A kind of preparation method of 5-chloro-2,3-dihydro-1-indanone
A technology of dihydro and indanone, which is applied in the field of medicine and chemical industry, can solve the problems of difficult post-processing, inconvenient operation, high energy consumption, etc., and achieve the effects of reducing the formation of high-temperature polymers, increasing the yield, and reducing the reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
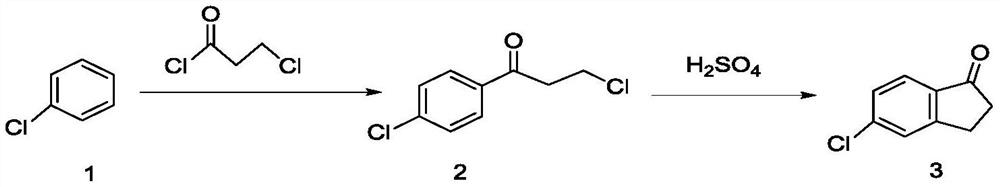
[0048] Throw 225g (2.0mol) of chlorobenzene into a 1000mL four-necked bottle, throw 332g (2.5mol) of aluminum trichloride under stirring, add 254g of 3-chloropropionyl chloride dropwise at 30°C, react for 3 hours, and then add it to the molten The mass ratio is 6:3.5:0.5 in aluminum trichloride, sodium chloride, potassium chloride mixture 332g. The temperature was raised to 110° C. for 3 hours, the conversion rate was 97%, and the reaction was completed. The reaction feed solution was added to ice water for hydrolysis, filtered, and dried to obtain 330.2 g of a brown solid, which was refined to obtain 249.3 g of light yellow powder 5-chloro-2,3-dihydro-1-indanone, and the content was detected by high pressure liquid chromatography It is 99.2%, and the yield is 74.8%.
Embodiment 2
[0050]Throw 225g (2.0mol) of chlorobenzene into a 1000mL four-necked bottle, throw 332g (2.5mol) of aluminum trichloride under stirring, add 254g of 3-chloropropionyl chloride dropwise at 30°C, react for 3 hours, and then add it to the molten The mass ratio is 6.5:3:0.5 in aluminum trichloride, sodium chloride, potassium chloride mixture 332g. The temperature was raised to 130° C. for 3 hours, the conversion rate was 98%, and the reaction was completed. The reaction feed solution was added to ice water for hydrolysis, filtered, and dried to obtain 335 g of a brown solid, which was refined to obtain 250.1 g of light yellow powder 5-chloro-2,3-dihydro-1-indanone, and the content detected by high pressure liquid chromatography was 99.5%, yield 75%.
Embodiment 3
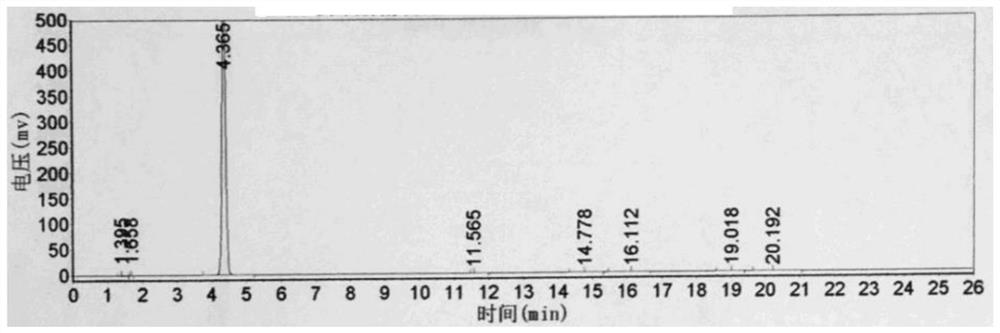
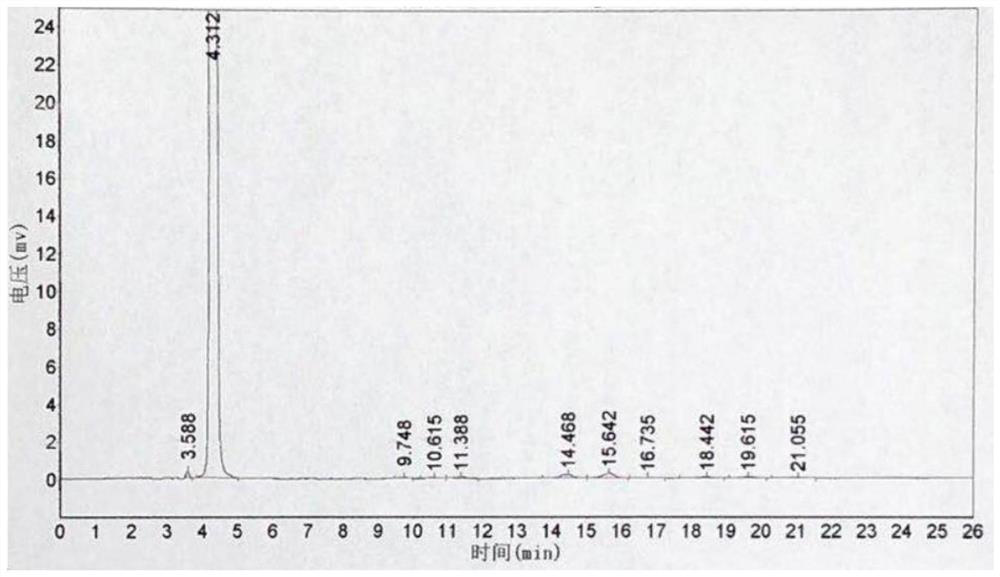
[0052] Throw 225g (2.0mol) of chlorobenzene into a 1000mL four-necked bottle, throw 332g (2.5mol) of aluminum trichloride under stirring, add 254g of 3-chloropropionyl chloride dropwise at 30°C, react for 3 hours, and then add it to the molten Remove in 332g of aluminum trichloride and sodium chloride mixture with a mass ratio of 7:3. The temperature was raised to 150° C. for 3 hours, the conversion rate was 95%, and the reaction was completed. The reaction feed solution was added to ice water for hydrolysis, filtered, and dried to obtain 335 g of a brown solid, which was refined to obtain 217.4 g of light yellow powder 5-chloro-2,3-dihydro-1-indanone, and the content detected by high pressure liquid chromatography was 99.3%, yield 65.2%. see figure 1 and figure 2 , figure 1 It is the HPLC of 5-chloro-2,3-dihydro-1-indanone standard substance, figure 2 High performance liquid chromatogram of 5-chloro-2,3-dihydro-1-indanone prepared for Example 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com