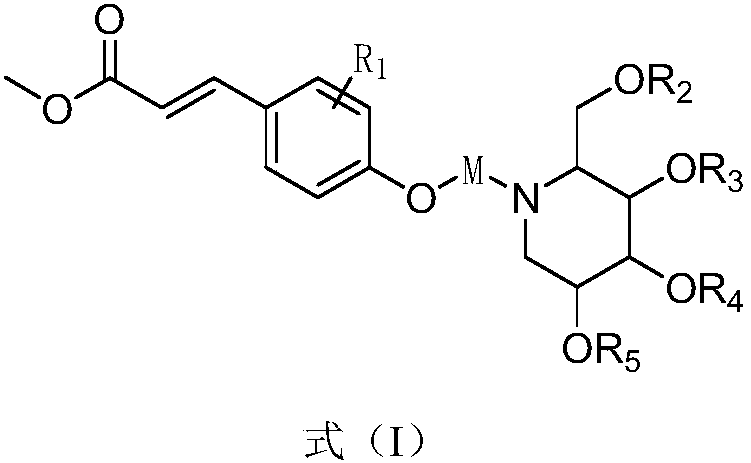
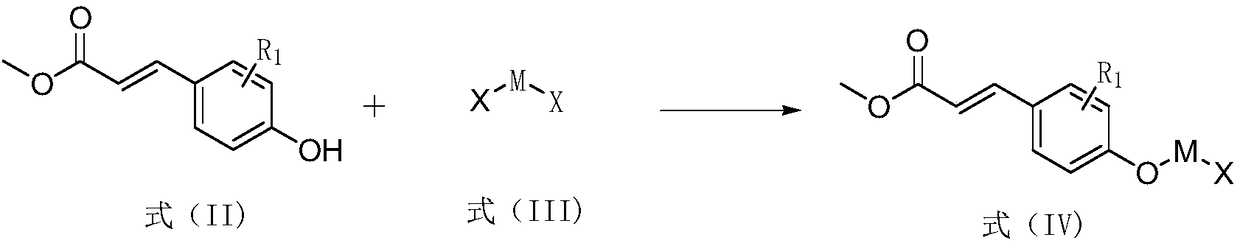1-Deoxynojirimycin-methyl hydroxycinnamate hybrid derivative and preparation method and application thereof
A technology of deoxynojirimycin and derivatives, which is applied in the fields of medicine and food, can solve the problems of hindering disaccharide decomposition and general effect, and achieve the effect of reducing postprandial blood sugar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of 1-deoxynojirimycin-methyl hydroxycinnamate hybrid derivative DMC-2
[0040] (1) The intermediate preparation of the methyl hydroxycinnamate of the connecting bridge:
[0041]Add 4g (22.44mmol) methyl p-hydroxycinnamate and 8.4g (44.88mmol) 1,2-dibromoethane raw materials into a 250mL round bottom flask, then add 6g (44.88mmol) potassium carbonate and 100mL acetone, Stir and react at 65°C until the end of the reaction; after the end of the reaction, pour the above reaction solution into water, extract with 20 mL of ethyl acetate, repeat the extraction 3 times, and combine the organic phases; dry with anhydrous sodium sulfate, Then desolvation and mixing sample, adopt silica gel column chromatography to separate, eluent is sherwood oil / ethyl acetate=20:1, has made the p-hydroxycinnamic acid methyl ester that has inserted the connecting bridge of 2 carbon atoms Intermediate; 1 H NMR (500MHz, DMSO) δ7.68(d, J=8.7Hz, 2H), 7.63(d, J=16.0Hz, 1H), 7....
Embodiment 2
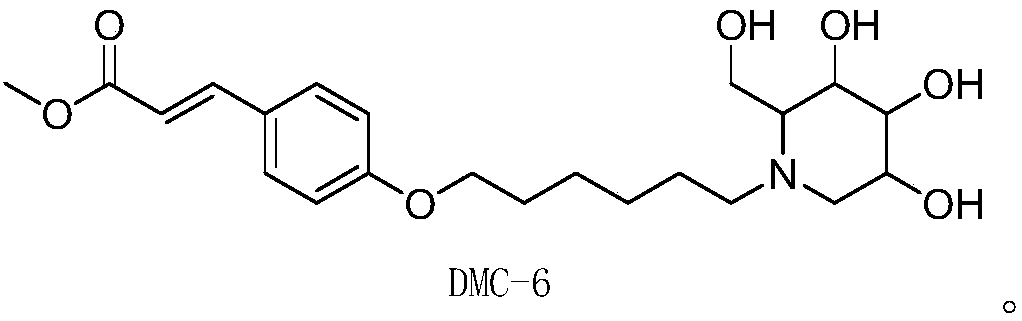
[0044] Example 2: Preparation of 1-deoxynojirimycin-methyl hydroxycinnamate hybrid derivative--DMC-6
[0045] (1) The intermediate preparation of the methyl hydroxycinnamate of the connecting bridge:
[0046] Add 4g (22.44mmol) methyl p-hydroxycinnamate and 10.95g (44.88mmol) 1,6-dibromohexane raw materials into a 250mL round bottom flask, then add 6g (44.88mmol) potassium carbonate and 100mL acetone, Stir and react at 65°C until the end of the reaction; after the end of the reaction, pour the above reaction solution into water, extract with 20 mL of ethyl acetate, repeat the extraction 3 times, and combine the organic phases; dry with anhydrous sodium sulfate, Then desolvation and mixing sample, adopt silica gel column chromatography to separate, eluent is sherwood oil / ethyl acetate=20:1, makes the p-hydroxycinnamic acid methyl ester that has inserted the connection bridge of 6 carbon atoms Intermediate; 1 H NMR (500MHz, DMSO) δ7.66(d, J=8.4Hz, 2H), 7.61(d, J=16.0Hz, 1H), 6...
Embodiment 3
[0049] Example 3: Preparation of 1-deoxynojirimycin-methyl hydroxycinnamate hybrid derivative DMC-5
[0050] (1) The intermediate preparation of the methyl hydroxycinnamate of the connecting bridge:
[0051] Add 4g (22.44mmol) methyl p-hydroxycinnamate and 10.31g (44.88mmol) 1,5-dibromopentane raw materials into a 250mL round bottom flask, then add 6g (44.88mmol) potassium carbonate and 100mL acetone, Stir and react at 65°C until the end of the reaction; after the end of the reaction, pour the above reaction solution into water, extract with 20 mL of ethyl acetate, repeat the extraction 3 times, and combine the organic phases; dry with anhydrous sodium sulfate, Then desolvation and mixing sample, adopt silica gel column chromatography to separate, eluent is sherwood oil / ethyl acetate=20:1, has made the p-hydroxycinnamic acid methyl ester that has inserted the connecting bridge of 5 carbon atoms Intermediate; 1 H NMR (500MHz, DMSO) δ7.66(d, J=8.7Hz, 2H), 7.61(d, J=16.0Hz, 1H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com