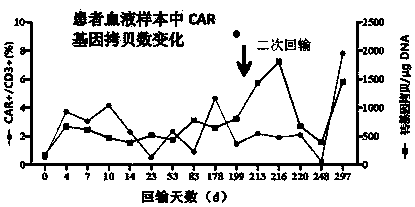
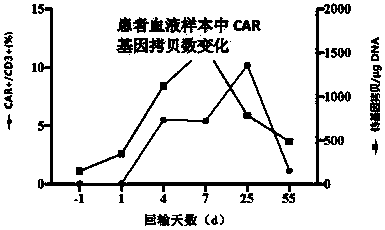
Chimeric antigen receptor and its gene and recombinant expression vector, CD19-CD20 dual targeting T cell and application thereof
A technology of chimeric antigen receptors and expression vectors, applied in the field of tumor biological products, can solve the problems of low efficiency, high risk of recurrence, and reduce the risk of recurrence, and achieve strong anti-tumor activity, avoid immune escape, and reduce the risk of recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The preparation method of the lentiviral expression vector is not particularly limited, and can be various methods that can be imagined by those skilled in the art. Preferably, the preparation method of the lentiviral vector includes the following steps:
[0040] (1) Amplify the hinge and transmembrane domains of CD8, the intracellular signal domain of CD137, and the intracellular signal domain of CD3ζ from T cell cDNA, and clone them into the vector pRRLsin (starter of lentiviral expression vector) Sub CMV promoter was replaced with EF1a promoter), pRRLsin-CD8-CD137-CD3ζ was constructed;
[0041] (2) Synthesize the nucleotide sequence encoding CD8a signal peptide and CD20ScFv-L-CD19ScFv, and clone them into pRRLsin-CD8-CD137-CD3ζ, and obtain the correct sequence of lentiviral expression vector pRRLsin-CD20ScFv-L after sequencing verification -CD19ScFv-CD8-CD137-CD3ζ.
[0042] In step (1), the method for amplifying the hinge and transmembrane domains of CD8, the intracellular...
Embodiment 1
[0084] This example is used to illustrate the preparation of T cells
[0085] (1) Take human venous blood in a vacuum tube containing heparin. Using lymphocyte separation fluid, mononuclear cells (PBMCs) were obtained by density gradient centrifugation.
[0086] (2) After washing PBMCs three times, use X-VIVO 15 containing 0.5% by volume of human autologous serum TM Adjust the final cell concentration of serum-free T cell culture medium to 2×10 6 Cells / mL; Inoculate the cells in 75cm pre-coated with retronectin with a final concentration of 10μg / mL and 50ng / ml CD3 monoclonal antibody 2 Cell culture flask. Then add recombinant human interleukin 2 with a final concentration of 300 U / mL to the medium, and the CO at 37°C and saturated humidity of 5% 2 Cultivate in an incubator.
[0087] (3) On the fourth day of culture, transfer the cells to an uncoated culture flask, and add T cell culture medium X-VIVO 15 according to the number of cells grown every 2 days TM , The control cell co...
Embodiment 2
[0089] This example is used to illustrate the construction of lentiviral expression vector
[0090] (1) Preparation of T cell cDNA
[0091] Centrifuge the T cells cultured in Example 1, extract the total RNA of the cells with the total RNA extraction kit RNAiso Reagent, and store them at -80°C for later use. RevertAid, a reverse transcription kit for extracted total RNA TM First Strand cDNA Synthesis Kit reverse transcribed T cell cDNA, stored at -20℃ for later use.
[0092] (2) Preparation of lentiviral expression vector (pRRLsin-CD20ScFv-L-CD19ScFv-CD8a-CD137-CD3ζ)
[0093] Design and synthesize the following primer sequences (wherein, the underlined mark is the protective base, and the box is the restriction site):
[0094]
[0095] Using the T cell cDNA in step (1) as a template, PCR amplification was performed with primers P1 and P2 to obtain the hinge region and transmembrane region of CD8 with a length of 227 bp. The nucleotide sequence is shown in SEQ ID NO. 4, and the two e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com