Synthesis method of dihydroxy rhodamine derivative or trihydroxy rhodamine derivative
A synthesis method and trihydroxyl technology, which are applied in chemical instruments and methods, organic chemistry, color-changing fluorescent materials, etc., can solve the problems of difficulty in introducing polymer chains, few external functional groups of rhodamine chromophore, etc., and achieve a simple and efficient synthesis route. The effect of simple and easy-to-obtain reaction substrates and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
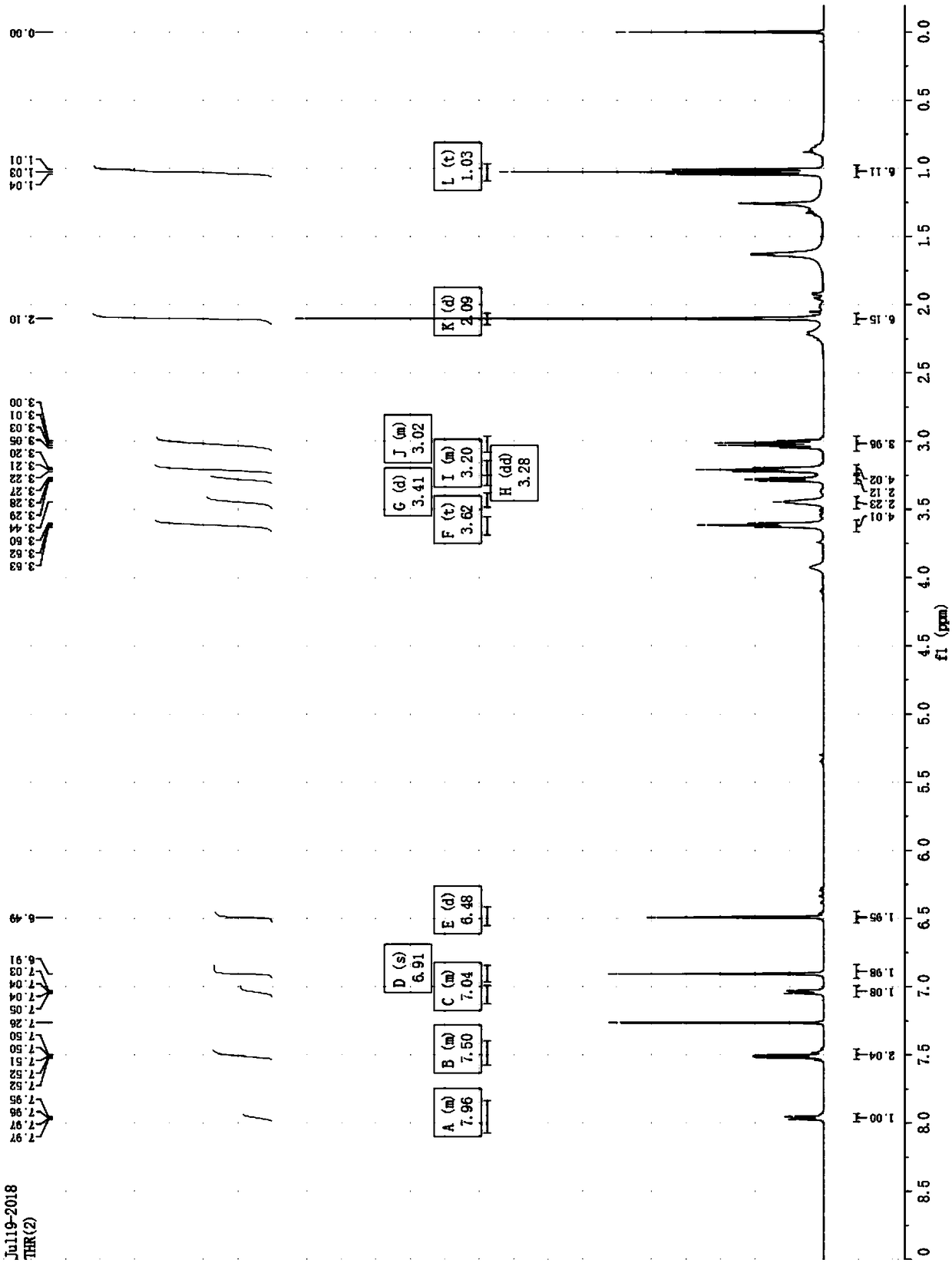
[0030] Take a 150mL round-bottomed flask, add Rh6G-OH (1.00g, 2.1mmol) and ethylene oxide (0.96g, 21.0mmol) into 70mL of glacial acetic acid in THF (1 / 1, v In / v), after stirring at room temperature for 48h, the solvent was removed by rotary evaporation to obtain a crude product. The crude product was separated by column chromatography (dichloromethane:ethanol=35:1, v / v) to obtain Rh6G-2OH (white solid) and Rh6G-3OH (pink solid). Overall yield: 72% (dihydroxy product: 0.40 g, 36%, trihydroxy product: 0.43 g, 36%).
example 2
[0032] Take a 150mL round-bottomed flask, add Rh6G-OH (1.00g, 2.1mmol) and ethylene oxide (0.96g, 21.0mmol) into 70mL of glacial acetic acid in THF (1 / 1, v In / v), after stirring at room temperature for 60 h, the solvent was removed by rotary evaporation to obtain a crude product. The crude product was separated by column chromatography (dichloromethane:ethanol=35:1, v / v) to obtain Rh6G-2OH (white solid) and Rh6G-3OH (pink solid). Overall yield: 75% (dihydroxy product: 0.17 g, 15%, trihydroxy product: 0.72 g, 60%).
example 3
[0034] Take a 150mL round-bottomed flask, add Rh6G-OH (1.00g, 2.1mmol) and ethylene oxide (0.96g, 21.0mmol) into 70mL of glacial acetic acid in THF (1 / 1, v In / v), after stirring at room temperature for 72h, the solvent was removed by rotary evaporation to obtain a crude product. The crude product was separated by column chromatography (dichloromethane:ethanol=35:1, v / v) to obtain Rh6G-3OH (pink solid). All trihydroxy product: 0.95 g, 80%.
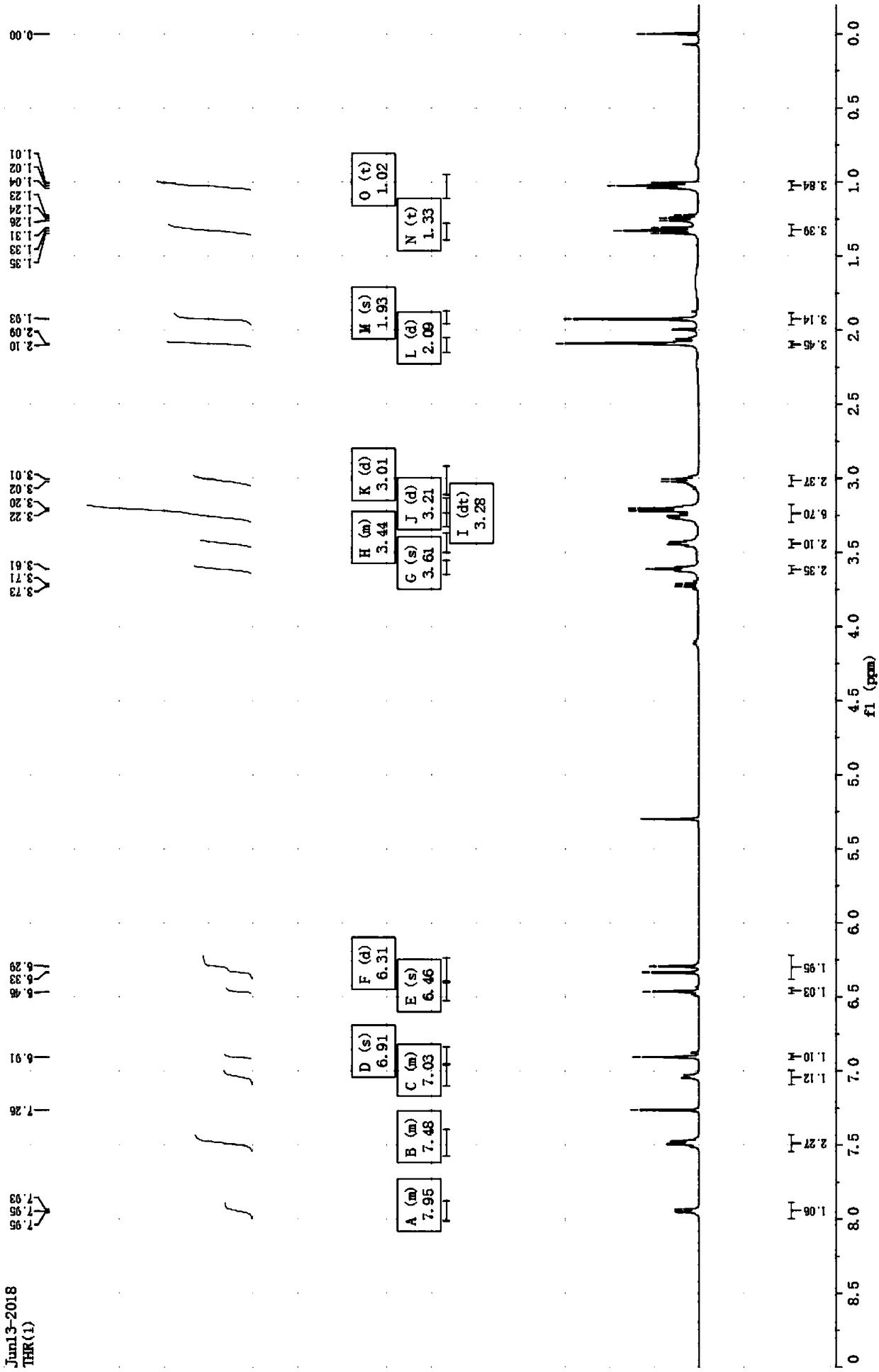
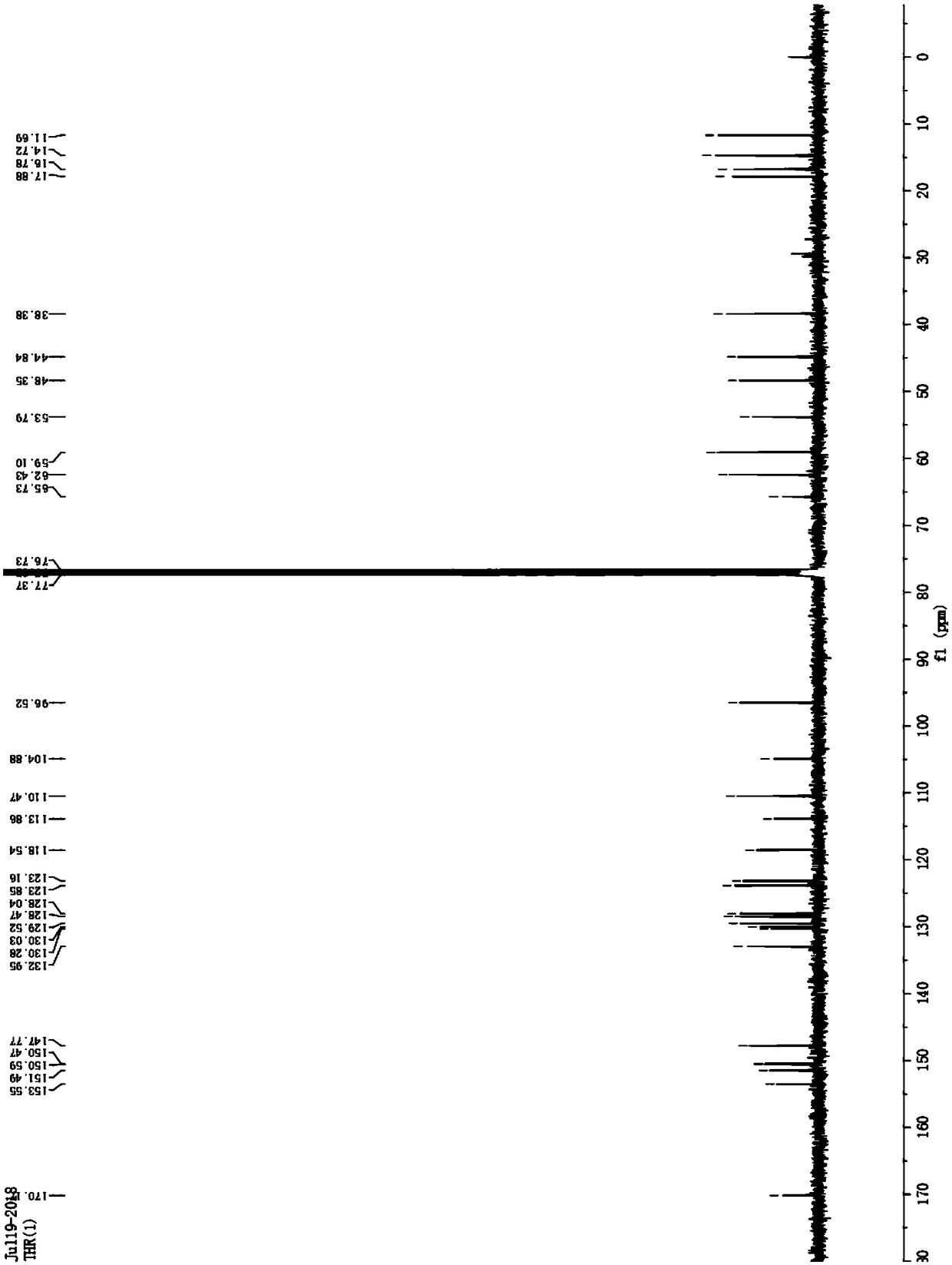
[0035] Rh6G-2OH
[0036] H NMR 1 H NMR (400MHz, CDCl 3 )δ / ppm: 8.07–7.83(m,1H), 7.57–7.40(m,2H),7.13–6.99(m,1H),6.91(s,2H),6.48(d,J=10.4 Hz,2H) ,3.62(t,J=5.3Hz,4H),3.41(d,J=25.5Hz,2H),3.28(dd,J=9.4,4.7Hz,2H),3.25–3.14(m,4H),3.08– 2.96(m,4H),2.09(d,J=9.1Hz,6H),1.03(t,J=7.1Hz,6H).
[0037] C NMR 13 C NMR (101MHz, CDCl 3 )δ / ppm:170.14,153.52, 151.46,150.56,150.44,147.74,132.92,130.25,130.00,129.49,128.44, 128.01,123.82,123.13,118.51,113.83,110.44,104.85,96.49,65.70, 62.40,59.07,53.76 ,48.32,44.81,38.35,17.85,16.75,14.69,11.66.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com