Detection method for rapidly and quantitatively detecting content of antinuclear antibody
An anti-nuclear antibody and detection method technology, applied in the field of rapid quantitative detection of anti-nuclear antibody content, can solve the problems of inaccurate quantification, false positive target antigen, membrane strip pollution, etc., to help monitor the disease and curative effect, predict the occurrence of and prognosis, the effect of accurate quantitative detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
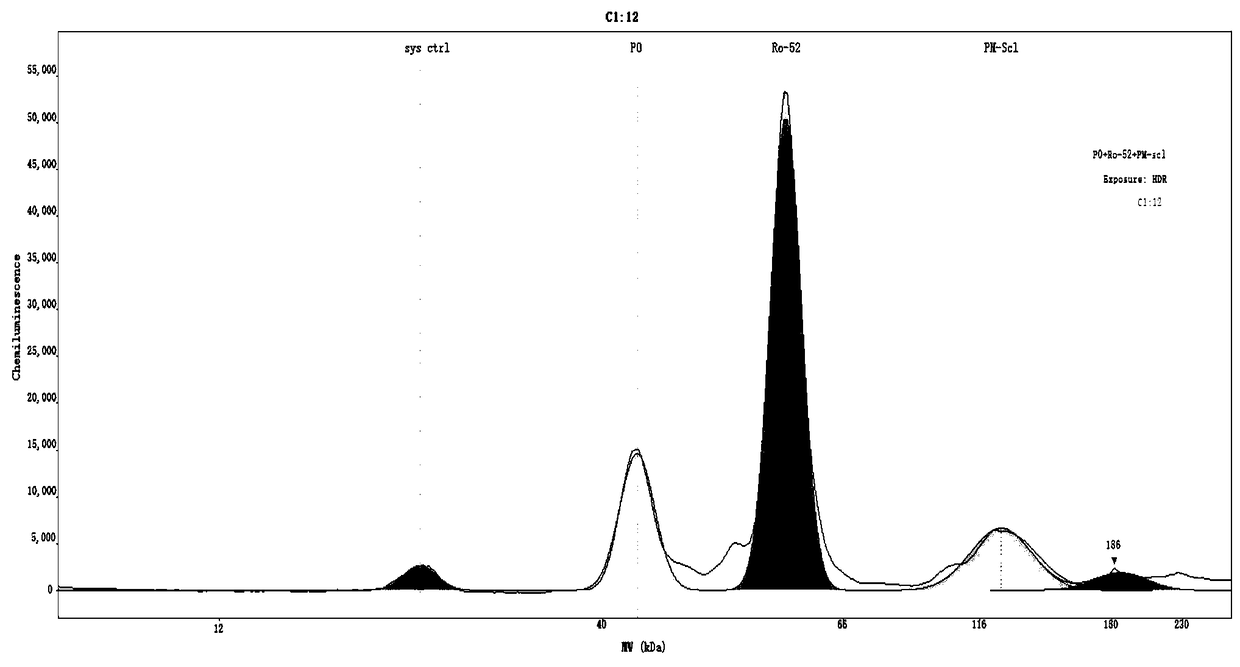
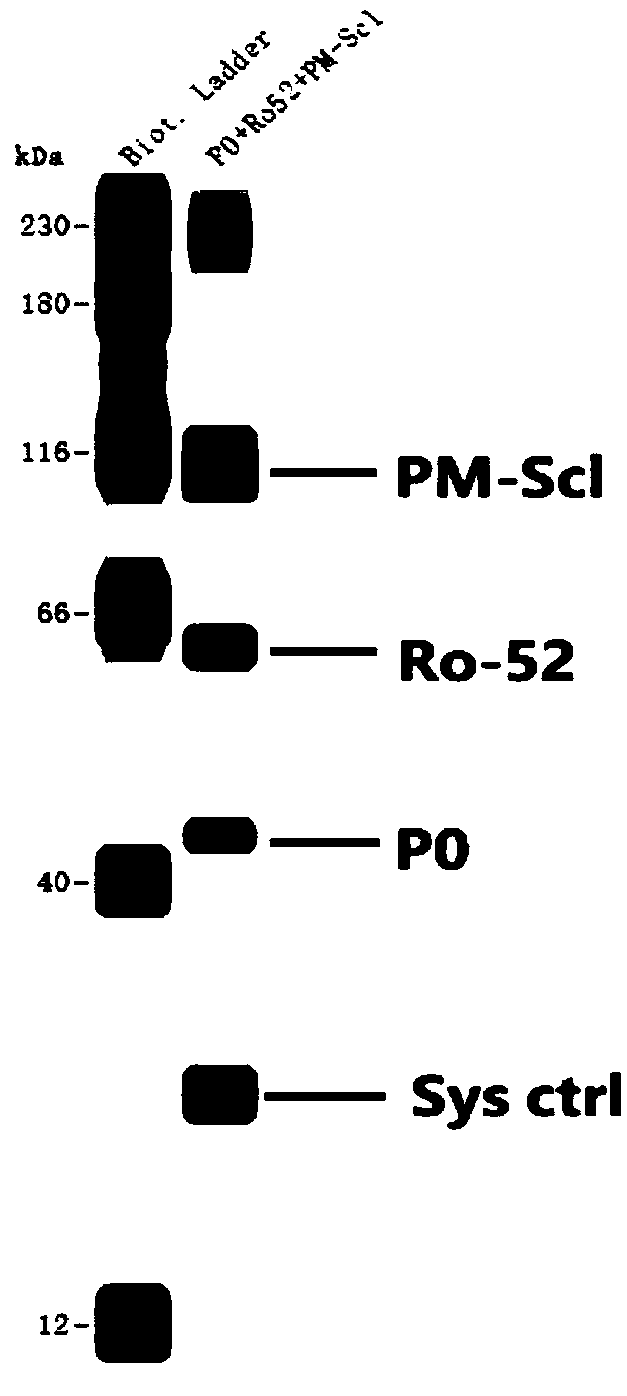
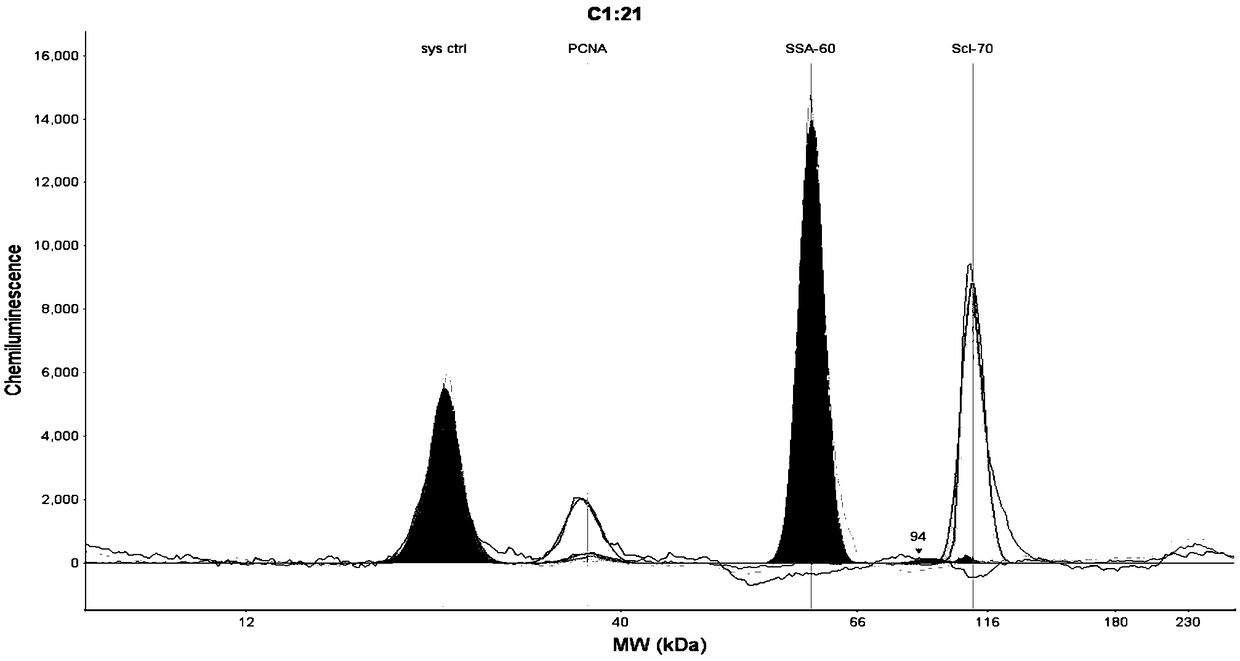
[0054] Step 1. Sample layout of Wes test board
[0055] The components of the detection reaction are pre-added to the detection plate of the Wes system, and the detection reaction is carried out in the matching cartridge containing 25 capillaries.
[0056] The layout of the samples in the test plate (25 wells / row, 1-25 wells from left to right) is as follows:
[0057] The first line, line A: A1 is the molecular weight standard, A2-A25 is the multi-combination target antigen sample;
[0058] The second line, line B: all are blocking solutions;
[0059] The third row C: C1 is the blocking solution, C2-C25 are the diluted serum samples;
[0060] The fourth row D: D1 is HRP-labeled streptavidin-HRP, D2-D25 is the secondary antibody-HRP mixture;
[0061] The fifth line, line E: all are luminescent substrate chromogenic solutions;
[0062] The sixth line, line F: empty.
[0063] Blocking solution, Streptavidin-HRP, and luminescent substrate chromogenic solution were equipped wi...
Embodiment 2
[0081] Step 1. Normalization processing of quantitative data
[0082] The peak area value (peakarea value, Pav) of the optical signal product peak area value (peakarea value, Pav) of the internal standard polypeptide control antigen sys ctrl in each capillary was 65,000, which was used as the control internal reference for normalizing the data of each antinuclear antibody (ANA). The formula for calculating the peak value of the ANA normalized product in serum samples is as follows:
[0083]
[0084] The normalized Pav value can be used as a quantitative standard unit for the antibody content level in serum.
[0085] Step 2. Establish the sample dilution factor
[0086]As the concentration of antinuclear antibody increases, the signal intensity of the luminescent substrate increases, but limited by the detection sensitivity and detection dynamic range of the detection system for the brightness of the band, the detection results of samples with too low or too high levels are...
Embodiment 3
[0098] Step 1. Optimal screening positive cut-off value (Cut-off value)
[0099] Receiver operator characteristic curve (ROC curve) is mainly used to select the best signal detection model or set the best threshold in the same model. In this embodiment, the ROC curve is used to set the best threshold.
[0100] Adopt the operating steps of Example 1, with the normalization processing method of Example 2, the sample dilution factor is taken 1:100, obtain the signal-to-noise ratio of six antinuclear antibodies in the patient sample (positive) and the control sample (negative) respectively S / N value and normalized Pav value.
[0101] Use SPSS data processing software to draw the ROC curve. The specific method is to use the Pav value as the data column, the positive and negative samples as the status column, and use "positive" as the positive status value to draw the ROC curve, and give many Cut- Sensitivity parameters and 1-specificity parameters corresponding to the off value, u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com