Preparation method and product of palbociclib
A solvent and intermediate technology, applied in the field of preparation of Palbociclib, can solve the problems of low yield of target products, unfavorable cost control, low environmental friendliness, etc., to improve the utilization rate of raw materials, reduce the difficulty of baking materials, reduce the The effect of product loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
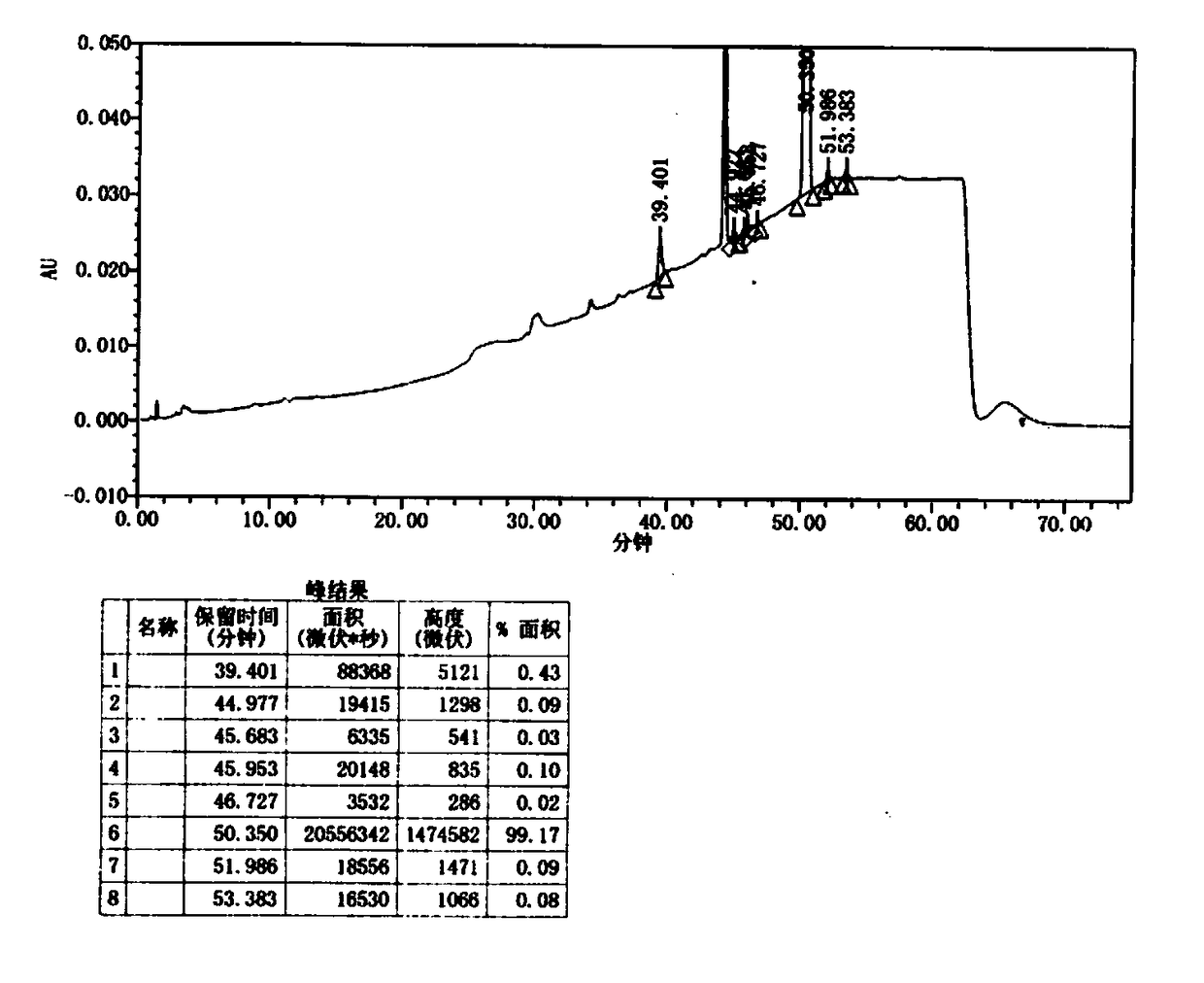
Image
Examples
Embodiment 1
[0039] The preparation method of palbociclib has the following steps:
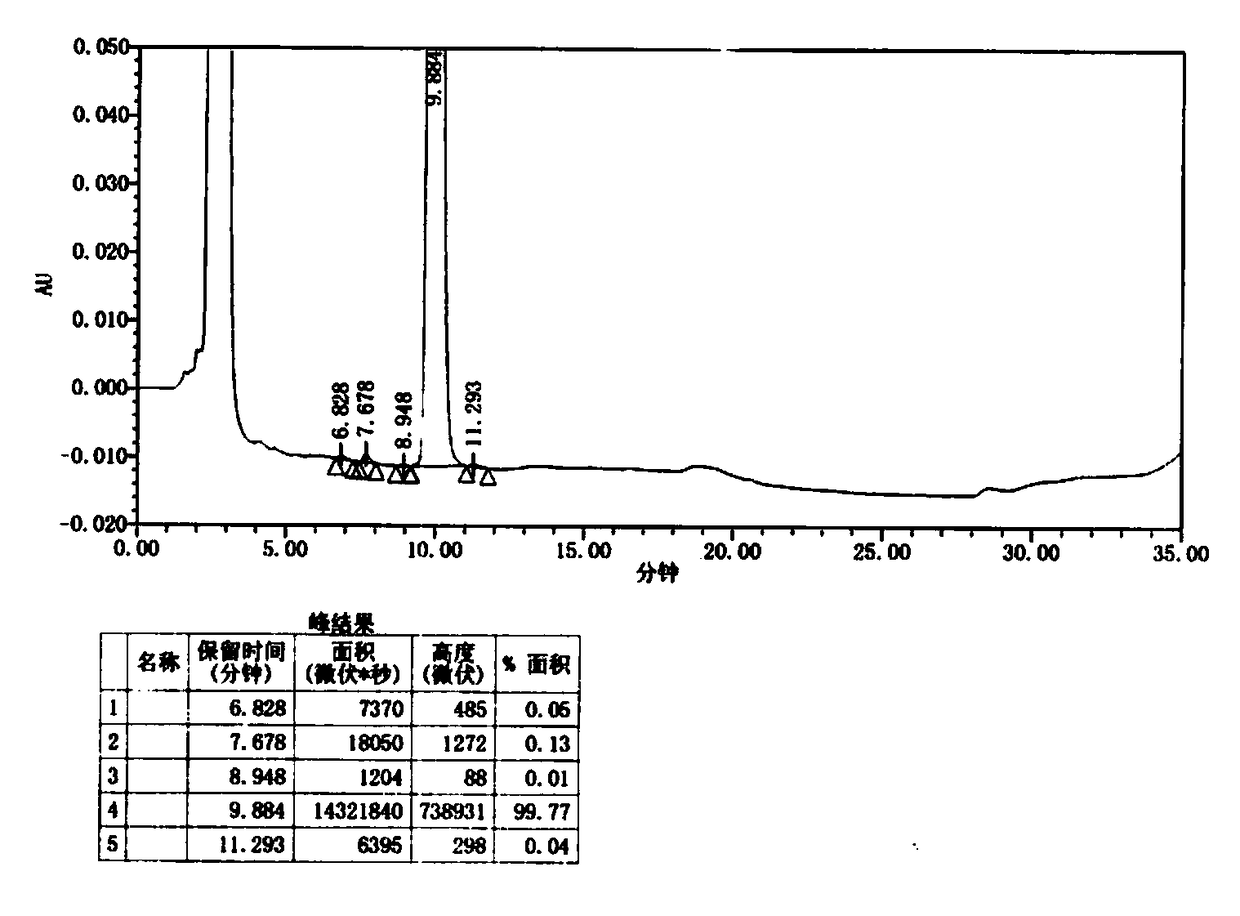
[0040] 1) Use inert gas N 2 Replace the reaction flask three times, add 1140g of tetrahydrofuran into the reaction flask, then add 93g of tert-butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate, cool to 0°C, add 280g of isopropyl Magnesium chloride, add dropwise within 1 hour, activate for 30 minutes; continue to add 6-bromo-2-chloro-8-cyclopentyl-5-methyl-pyrido[2,3-D]pyrimidine-7(8H) - 95g of ketone, the dropwise addition was completed within 1 hour, and the temperature was raised to 60°C for 30 minutes to react, until the end of the reaction, the acetic acid / THF mixture was added dropwise (the mass ratio of acetic acid to THF was 1:10), and the pH of the solution was adjusted to precipitate a solid. Cool down to 0°C, filter, take the filter cake and dry to obtain 139 g of intermediate I as a yellow solid powder, with a yield of 85.8%. The HPLC spectrum of the obtained palbociclib intermediate I is ...
Embodiment 2
[0044] The preparation method of palbociclib has the following steps:
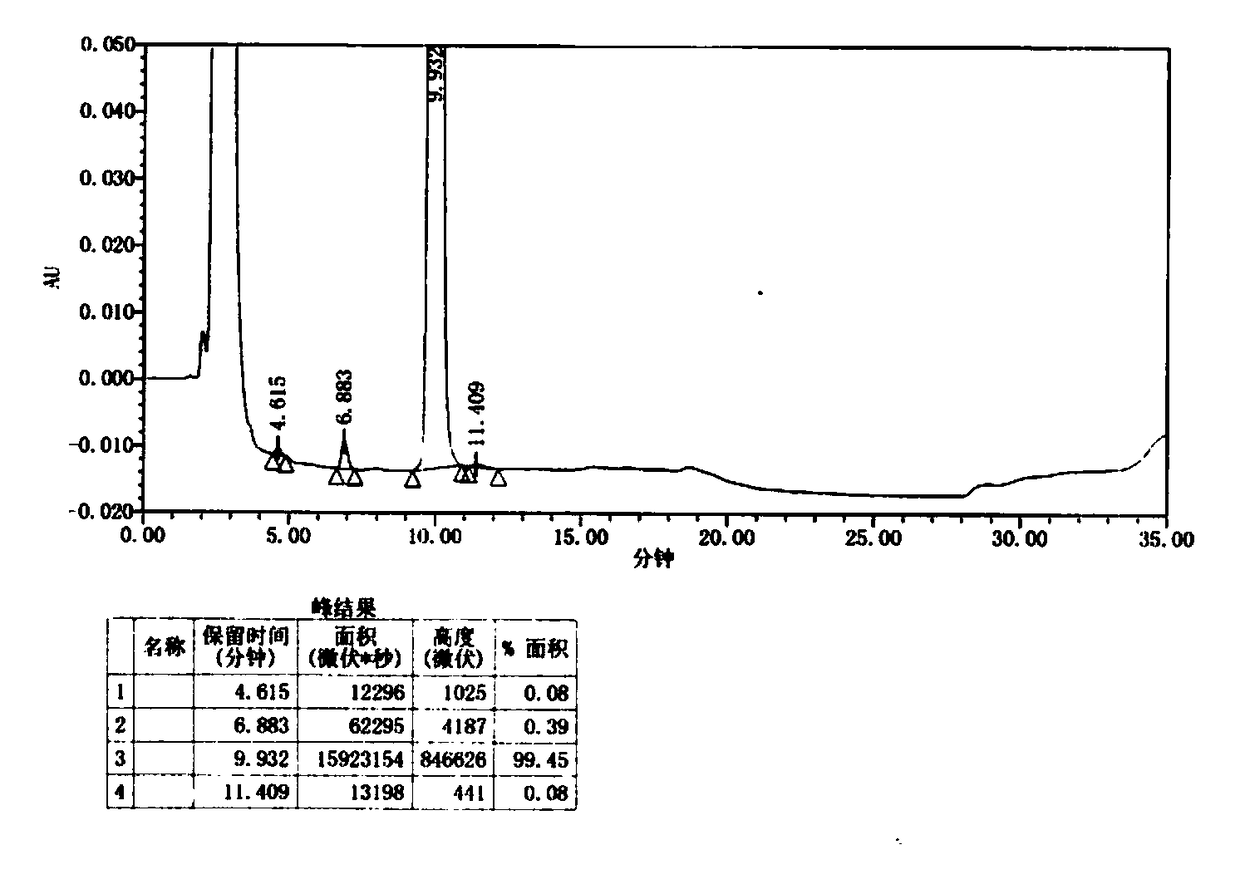
[0045] 1) Use inert gas N 2 Replace the reaction flask three times, add 1140g of toluene into the reaction flask, then add 93g of tert-butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate, cool to 20°C, add 140g of isopropyl Magnesium chloride, added dropwise within 1 hour, activated for 30 minutes; continue to add 6-bromo-2-chloro-8-cyclopentyl-5-methyl-pyrido[2,3-D]pyrimidine-7(8H )-ketone 95g, dropwise added within 1 hour; continue to dropwise add 140g isopropylmagnesium chloride, dropwisely completed within 1 hour, be warming up to 60 ℃ and react for 30 minutes, to the end of reaction, by dropwise adding acetic acid / tetrahydrofuran mixture ( The mass ratio of acetic acid to tetrahydrofuran is 1:10), adjust the pH of the solution to precipitate a solid, cool down to 0°C, filter, take the filter cake and dry to obtain 141 g of yellow solid powder intermediate I, with a yield of 87%. The HPLC spectrum ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com