Method for preparing canthaxanthin by beta-carotene
A technology of carotene and canthaxanthin, which is applied in the field of synthesis of canthaxanthin by one-step oxidation method, can solve hidden dangers and safety problems, and achieve the effects of low dosage, high reaction yield and safe process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
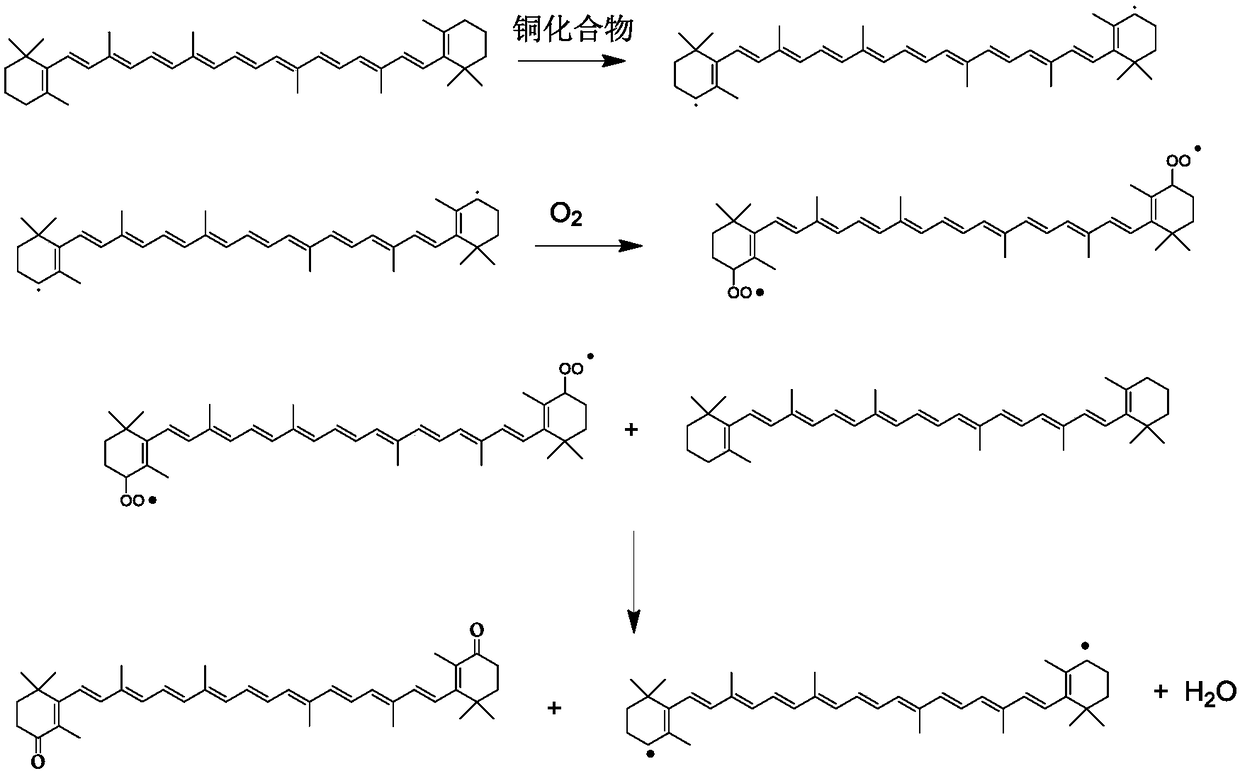
[0038] In the autoclave of 1L, add 16.08g (0.03mol) β-carotene successively, 800g dichloroethane, 4mg (0.1%, mol ratio is relative to β-carotene) copper chloride, 2.2mg (0.1%, mol Relative to β-carotene) glycine, 68mg (0.2%, molar ratio relative to β-carotene) β-cyclodextrin, filled with air and pressurized to an absolute pressure of 2Mpa, heated to 60°C to react, reacted for 15h, and the reaction solution Take a small amount for high-performance liquid chromatography analysis, the reaction conversion rate is 95.6%, and the selectivity is 93.6%. Stop the reaction, filter the reaction solution to remove the catalyst, then heat the filtrate to reflux, add 800 g of ethanol to the system, cool to -10°C and filter with suction to obtain 13.2 g of canthaxanthin solids, with a yield of 82.1%.
Embodiment 2
[0040] Add 16.08g (0.03mol) beta-carotene successively in the autoclave of 1L, 640g dichloroethane, 0.05% (molar ratio is relative to beta-carotene) copper acetate, 0.2% (molar ratio is relative to beta-carotene) element) glycine, 0.2% (molar ratio relative to β-carotene) β-cyclodextrin is filled with air and pressurized to an absolute pressure of 2Mpa, heated to 50°C for reaction, and reacted for 15 hours. Take a small amount of the reaction solution for high-performance liquid chromatography analysis , the reaction conversion rate is 92.6%, and the selectivity is 96.6%. Stop the reaction, filter the reaction solution to remove the catalyst, then heat the filtrate to reflux, add 600 g of ethanol to the system, cool to -20°C and filter with suction, the yield of canthaxanthin is 81.6%.
Embodiment 3
[0042] In the autoclave of 1L, add 16.08g (0.03mol) β-carotene successively, 640g dichloroethane, 0.05% (molar ratio is relative to β-carotene) copper trifluoroacetate, 0.2% (molar ratio is relative to β-carotene) -carotene) serine, 0.2% (molar ratio relative to β-carotene) α-cyclodextrin is filled with air and pressurized to an absolute pressure of 1.5Mpa, heated to 70°C for reaction, and reacted for 12 hours. Take a small amount of the reaction solution for high-efficiency liquid According to phase chromatography analysis, the reaction conversion rate is 91.6%, and the selectivity is 95.6%. Stop the reaction, filter the reaction solution to remove the catalyst, then heat the filtrate to reflux, add 800 g of ethanol to the system, cool to 0° C. and suction filter, the yield of canthaxanthin is 79.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com