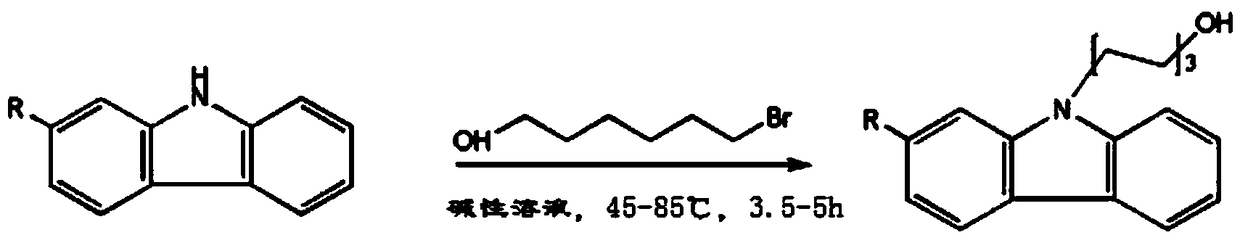
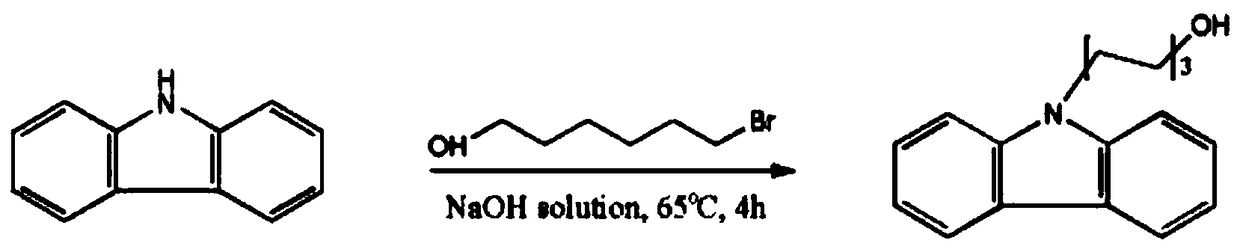
N-(6-hydroxyl hexyl) carbazole derivatives and synthesis method of N-(6-hydroxyl hexyl) carbazole and its derivative
A technology of carbazole derivatives and synthetic methods, applied in the direction of organic chemistry, can solve the problems of lower final product yield, increased synthesis cost, long reaction time, etc., and achieve convenient separation and purification, good tolerance and universality, The effect of high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1, a kind of synthetic method of N-(6-hydroxyhexyl) carbazole is carried out according to the following steps: add carbazole 1.67g (10mmol) successively in single-necked flask, benzyltriethylammonium chloride 150mg, two Methyl sulfoxide 20ml, saturated sodium hydroxide 15ml, 6-bromo-n-hexanol 1.57ml (12mmol), react at 65°C for 4h under magnetic stirring. Add 100ml of deionized water and 100ml of ethyl acetate, separate the liquids, wash the organic phase with water several times until the solution is neutral (generally 3 times), separate the organic phase and add anhydrous sodium sulfate to dry, filter, and remove ethyl acetate by rotary evaporation under reduced pressure Esters, and the residue was separated by silica gel column chromatography (eluent: dichloromethane) to obtain 2.54 g of white solid N-(6-hydroxyhexyl)carbazole with a yield of 95%. Synthetic route such as figure 2 shown.
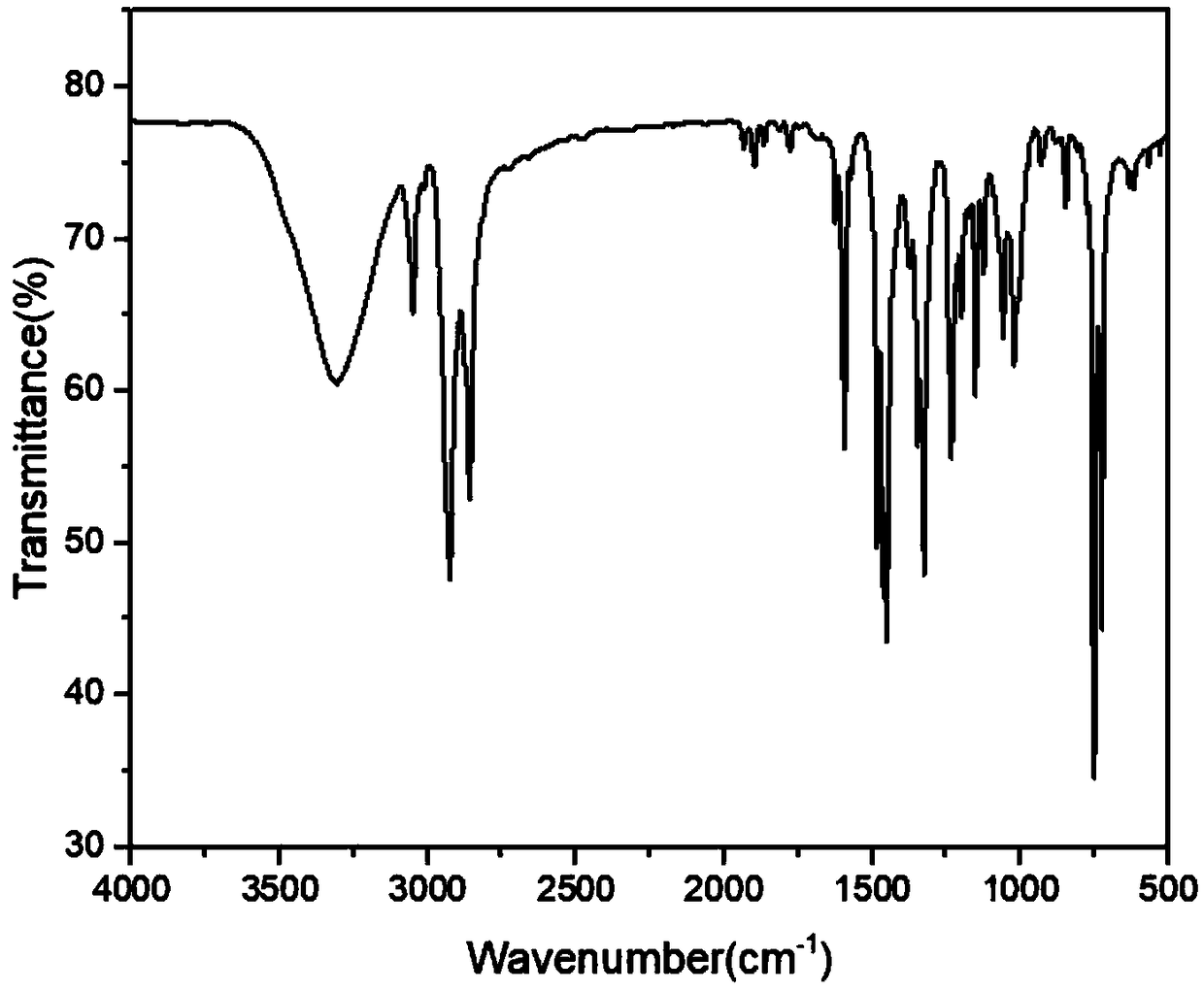
[0028] Product N-(6-hydroxyhexyl) carbazole characterization data ...
Embodiment 2
[0034] Embodiment 2, a kind of synthetic method of N-(6-hydroxyhexyl) carbazole is carried out as follows: add carbazole 1.67g (10mmol) successively in single-necked flask, tetrabutylammonium bromide 75mg, N, N - Dimethylformamide 15ml, saturated potassium hydroxide 10ml, 6-bromo-n-hexanol 10.02mmol, react at 45°C for 3.5h under magnetic stirring. Add 100ml of deionized water and 100ml of ethyl acetate, separate the liquids, wash the organic phase with water several times until the solution is neutral (generally 3 times), separate the organic phase and add anhydrous sodium sulfate to dry, filter, and remove ethyl acetate by rotary evaporation under reduced pressure Esters, and the residue was separated by silica gel column chromatography (eluent: dichloromethane) to obtain 2.14 g of white solid N-(6-hydroxyhexyl)carbazole with a yield of 50%.
Embodiment 3
[0035] Embodiment 3, a kind of synthetic method of N-(6-hydroxyhexyl) methoxycarbazole, carries out according to the following steps:
[0036] Add 1.97g (10mmol) of methoxycarbazole, 150mg of benzyltriethylammonium chloride, 20ml of dimethyl sulfoxide, 15ml of saturated sodium hydroxide, and 1.57ml (12mmol) of 6-bromo-n-hexanol in a single-necked flask. , Reacted at 65°C for 4h under magnetic stirring. Add 100ml of deionized water and 100ml of ethyl acetate, separate the liquids, wash the organic phase with water several times until the solution is neutral (generally 3 times), separate the organic phase and add anhydrous sodium sulfate to dry, filter, and remove ethyl acetate by rotary evaporation under reduced pressure Esters, and the residue was separated by silica gel column chromatography (eluent: dichloromethane) to obtain 2.7 g of white solid N-(6-hydroxyhexyl)methoxycarbazole with a yield of 90%. Synthetic route such as Figure 4 shown.
[0037] Product N-(6-hydroxyh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com