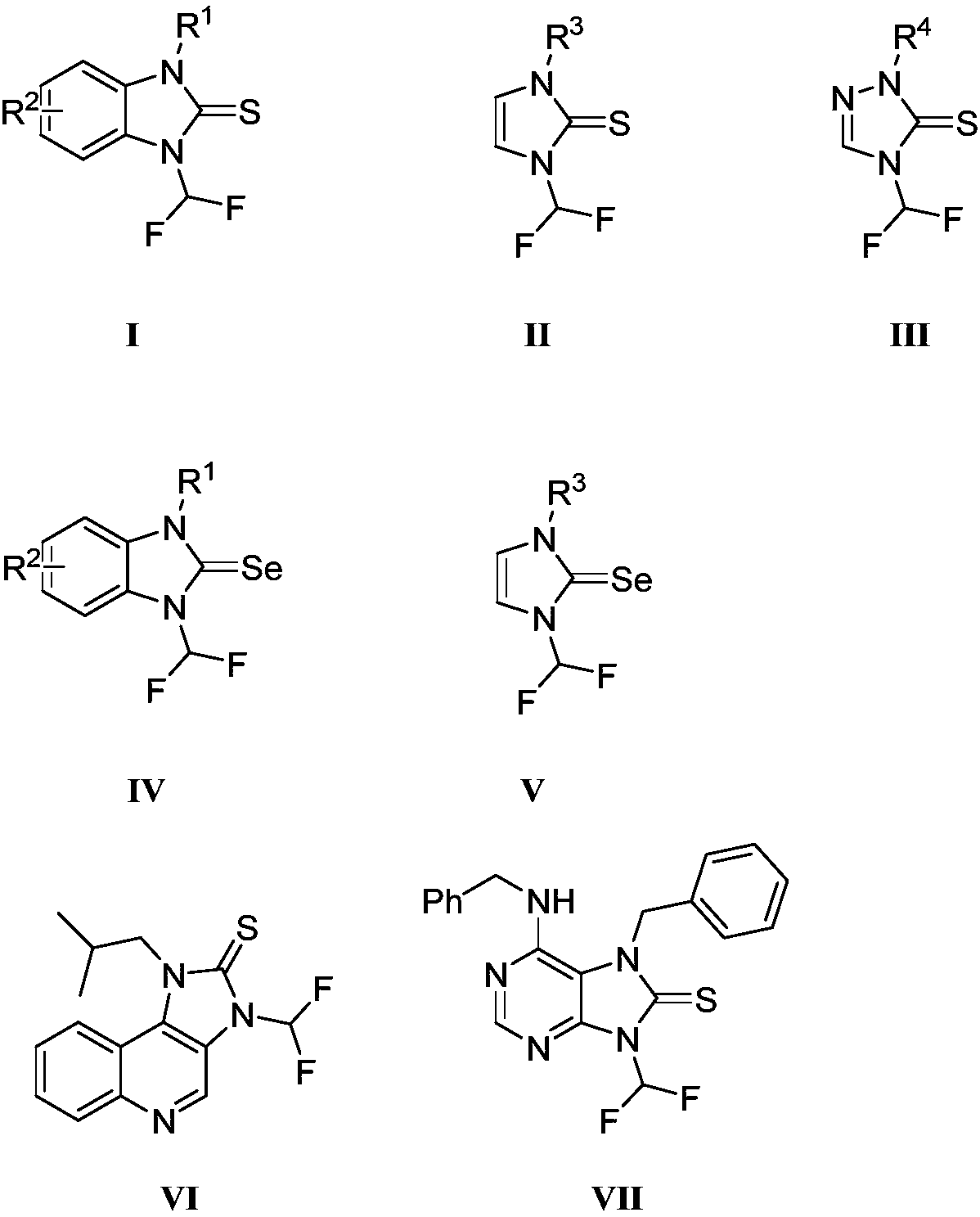
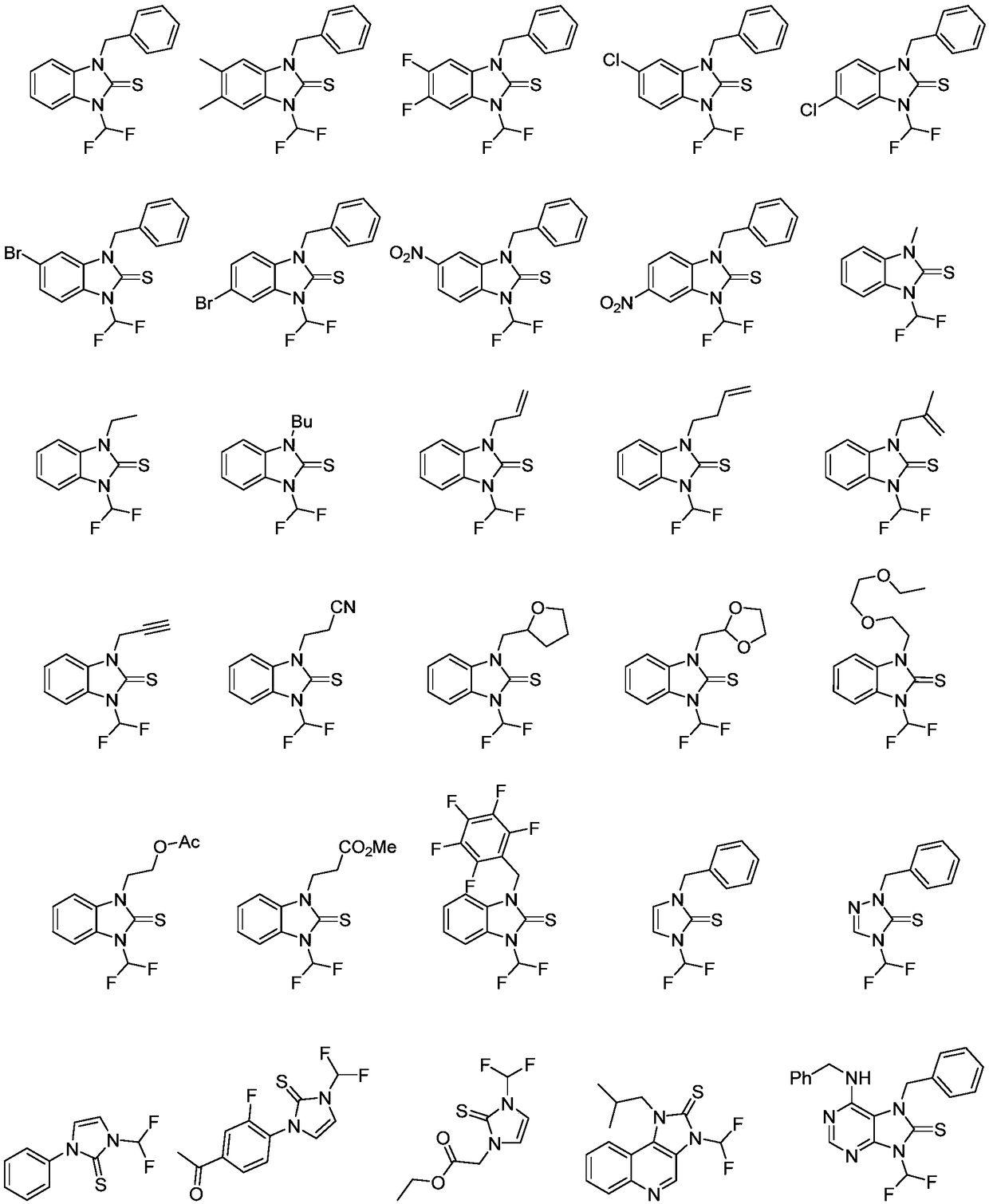
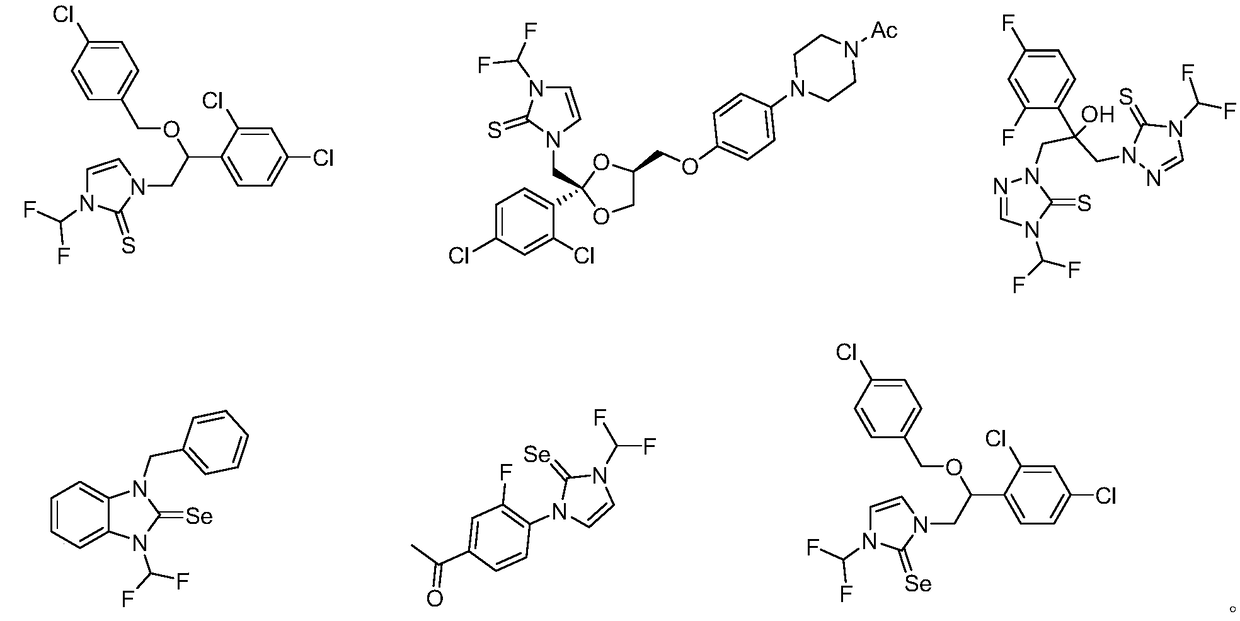
N-difluoromethylazole sulfur (selenium) urea derivative and preparation method thereof
A technology of difluoromethyl imidazole thiourea and difluoromethyl triazole thiourea, which is applied in the field of N-difluoromethyl azole thiourea derivatives and its preparation, and can solve the problem of functional group damage, difficulty in obtaining, unfavorable Problems such as the construction of drug molecular libraries, to achieve the effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Benzyl-3-(difluoromethyl)-1,3-dihydro-2H-benzo[d]imidazole-2-thione compound shown in preparation formula I (R1 is benzyl, R 2 for hydrogen):
[0037]
[0038] The synthetic steps of 1-benzylbenzimidazole:
[0039] In an oven-dried 100 ml round flask equipped with a magnetic stirrer, add benzimidazole (5.0 mmol, 0.59 g), benzyl bromide (6.0 mmol) and Cs 2 CO 3 (10.0 mmol). The reaction mixture was stirred with acetonitrile at 80°C under reflux for 8 hours. After the reaction was completed, the reaction mixture was cooled to room temperature. Acetonitrile was removed under vacuum to give a residue which was dissolved in dichloromethane and filtered to remove inorganic salts. The filtrate was concentrated in vacuo and the resulting residue was purified by flash column chromatography using petroleum and EtOAc as eluents in 92% (0.957 g) isolated yield.
[0040] Method 1: Add 1-benzylbenzimidazole (0.4 mmol), sulfur powder (S 8 ) (0.8mmol), ethyl bromodifluoroacet...
Embodiment 2
[0043] 1-(difluoromethyl)-3-methyl-1,3-dihydro-2H-benzo[d]imidazole-2-thione compound shown in preparation formula I (R 1 is methyl, R 2 for hydrogen):
[0044]
[0045] Add 1-methylbenzimidazole (0.4 mmol), sulfur powder (S 8 ) (0.8mmol), ethyl bromodifluoroacetate (1.0mmol) in sodium hydroxymethylsulfinate (HOCH 2 SO 2 Na) (0.8 mmol) was used as a catalyst in a solution of N,N-dimethylacetamide (DMA) (2.0 mL) and the reaction was stirred at 100°C for 24 hours. After the reaction, the reaction mixture was cooled to room temperature, the mixture was extracted with saturated brine and ethyl acetate, and the combined organic layers were washed with anhydrous Na 2 SO 4 Dry and evaporate the solvent in vacuo on a rotary evaporator. The crude mixture was purified by flash column chromatography using 300-400 mesh silica gel and monitored spot plate by preparative TLC to give the product (I-10) as a white solid, m.p.: 111.3-112.5°C, yield 73%.
Embodiment 3
[0047] 1-benzyl-3-(difluoromethyl)-1,3-dihydro-2H-imidazole-2-thione compound (R 1 for benzyl):
[0048]
[0049] Add 1-benzylimidazole (0.4 mmol), sulfur powder (S 8 ) (0.8mmol), ethyl bromodifluoroacetate (1.0mmol) in sodium hydroxymethylsulfinate (HOCH 2 SO 2 Na) (0.8 mmol) was used as a catalyst in a solution of N,N-dimethylacetamide (DMA) (2.0 mL) and the reaction was stirred at 100°C for 24 hours. After the reaction, the reaction mixture was cooled to room temperature, the mixture was extracted with saturated brine and ethyl acetate, and the combined organic layers were washed with anhydrous Na 2 SO 4 Dry and evaporate the solvent in vacuo on a rotary evaporator. The crude mixture was purified by flash column chromatography using 300-400 mesh silica gel and monitored spot plate by preparative TLC to give the product (I-24) as a light yellow solid, m.p.: 107.5-108.4°C, yield 37%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com