Intestinal flora capsule preparation method and intestine flora capsules
A technology of capsule preparation and enterobacteria capsule, which is applied in the field of microbiology, can solve the problems of long treatment cycle and achieve the effects of shortening the treatment cycle, increasing colon motility, and relieving pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The invention provides a preparation method of enterobacteria capsule, comprising the following steps:
[0051] Step S1, obtaining human feces, mixing human feces with sterile saline in a certain proportion to obtain a mixed solution;
[0052] Step S2, stirring the mixed solution to transform the mixed solution into a suspension;
[0053] Step S3, performing microfiltration on the suspension to obtain a filtrate;
[0054] Step S4, centrifuging the filtrate to obtain a precipitate;
[0055] Step S5, mixing the precipitate with the lyoprotectant in a certain proportion to obtain a pre-freeze solution, pre-freezing the pre-freeze solution and performing freeze-drying to obtain a lyophilized powder;
[0056] Step S6, adding the freeze-dried powder to the capsule, sealing and packaging to obtain the Enterobacteria capsule, and storing the Enterobacteria capsule at -20°C.
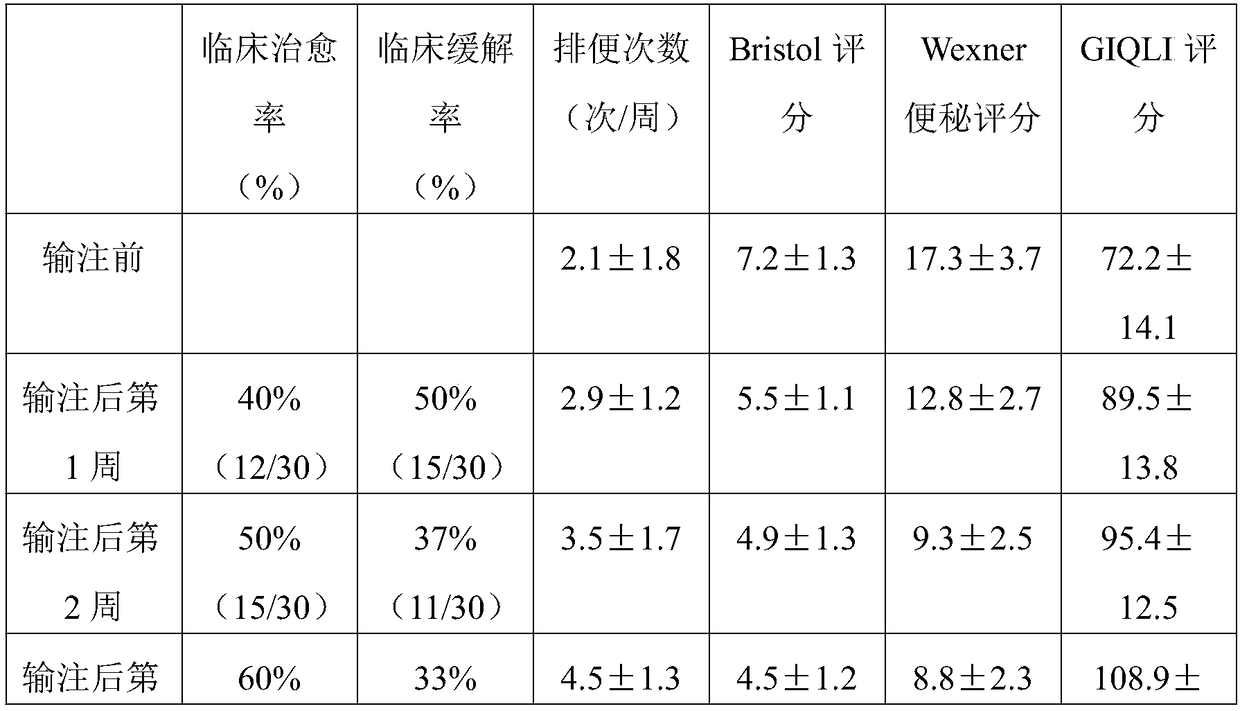
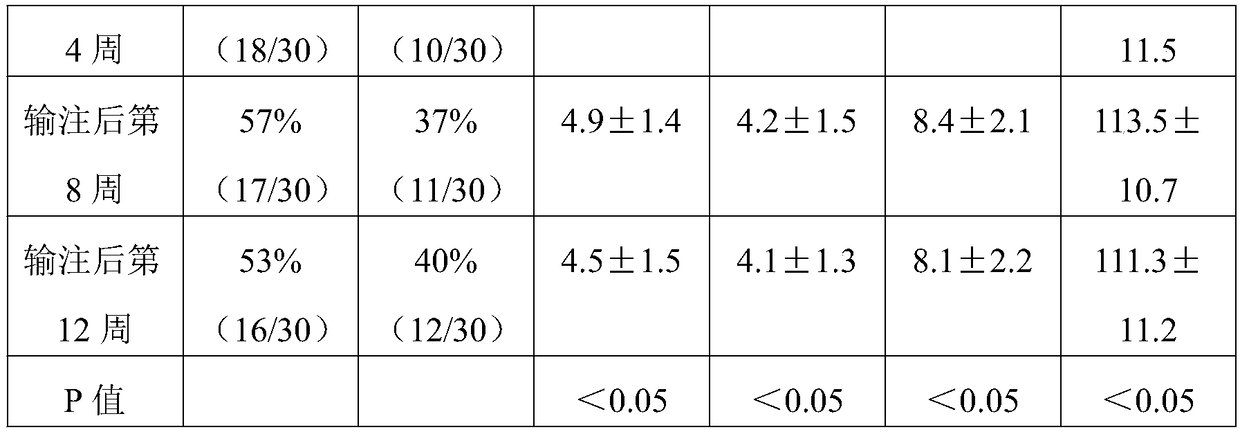
[0057] In the present invention, the active ingredient of the enteric bacteria capsule is sediment, ...
Embodiment 2
[0111] This embodiment is a specific embodiment of the present invention.
[0112] A preparation method of enterobacteria capsule, comprising the following steps:
[0113] Step S1. Obtain 100g of human feces, put it into a sterile mixing tank, pour 300ml of 0.9% sterile saline, and stir to obtain a mixed solution;
[0114] Step S2, stirring the mixed solution, so that the flora in human feces is released from the feces, so that the mixed solution becomes a suspension rich in flora;
[0115] Step S3, the suspension can be subjected to three-stage microfiltration treatment, so that the suspension passes through filter screens with apertures of 2.0mm, 0.5mm, and 0.2mm in sequence to obtain the filtrate from which residues are removed;
[0116] Step S4, perform low-temperature centrifugation on the filtrate, the working conditions are: temperature 4°C, rotation speed 2000rpm, centrifugation time 3min, remove the supernatant to obtain a precipitate, which is the enterobacteria com...
Embodiment 3
[0120] This embodiment is a specific embodiment of the present invention.
[0121] A preparation method of enterobacteria capsule, comprising the following steps:
[0122] Step S1: Obtain 100g of human feces, put it into a sterile mixing tank, pour 500ml of 0.9% sterile saline, stir and mix to obtain a mixed solution;
[0123] Step S2, stirring the mixed solution, so that the flora in human feces is released from the feces, so that the mixed solution becomes a suspension rich in flora;
[0124] Step S3, the suspension can be subjected to secondary microfiltration treatment, so that the suspension passes through filter screens with apertures of 2.0 mm and 0.5 mm respectively, to obtain a filtrate from which residues are removed;
[0125] Step S4, perform low-temperature centrifugation on the filtrate, the working conditions are: temperature 4°C, rotation speed 2500rpm, centrifugation time 5min, remove the supernatant to obtain a precipitate, which is the enterobacteria composi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Escherichia coli | aaaaa | aaaaa |
| Escherichia coli | aaaaa | aaaaa |
| Escherichia coli | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com