AID (activation-induced cytidine deaminase) mutant and application thereof
A mutant and carrier technology, applied in the fields of bioengineering and genetic engineering, can solve the problems of small library capacity, long time required for affinity maturation, loss of AID activity, etc., and achieve enhanced mutation ability, fast and efficient antibody affinity maturation, fast and efficient established effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1AI
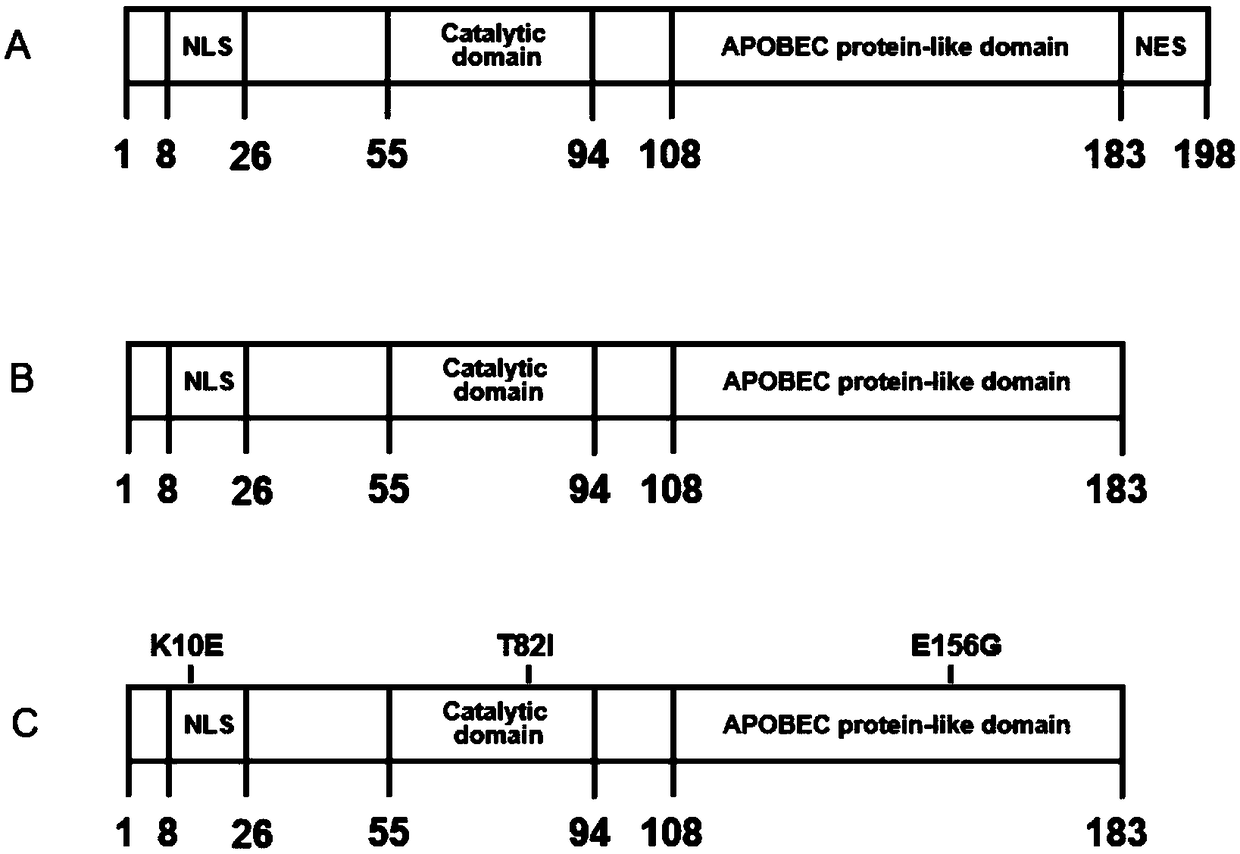
[0042] Embodiment 1 AID mutant construction
[0043] The present invention constructs four kinds of AID mutants, AID mutants with NES domain deleted: mouse source mAID-del (SEQ ID NO.5), human source hAID-del (SEQ ID NO.7); NES domain is deleted and contains AID mutant with K10E / T82I / E156G point mutation: mAID-del-K10E / T82I / E156G (abbreviated mAID-plus) (SEQ ID NO.9), hAID-del-K10E / T82I / E156G (abbreviated hAID-plus) (SEQ ID NO. 11). For a schematic diagram of the mutant structure, see figure 1 .
[0044] Wild-type AID: mAID (SEQ ID NO.1), hAID (SEQ ID NO.3) as control.
[0045] 1. Primer design
[0046] According to mAID (SEQ ID NO.2), hAID (SEQ ID NO.4), mAID-del (SEQ ID NO.6), hAID-del (SEQ ID NO.8), mAID-plus (SEQ ID NO.10 ), the nucleotide sequence of hAID-plus (SEQ ID NO.12), use software design PCR amplification primer as follows:
[0047] Primer name
Primer sequence
serial number
HindⅢ-mAID-F
GTACATAAGCTTATGGACAGCCTTCTG
SEQ ID NO.17...
Embodiment 2
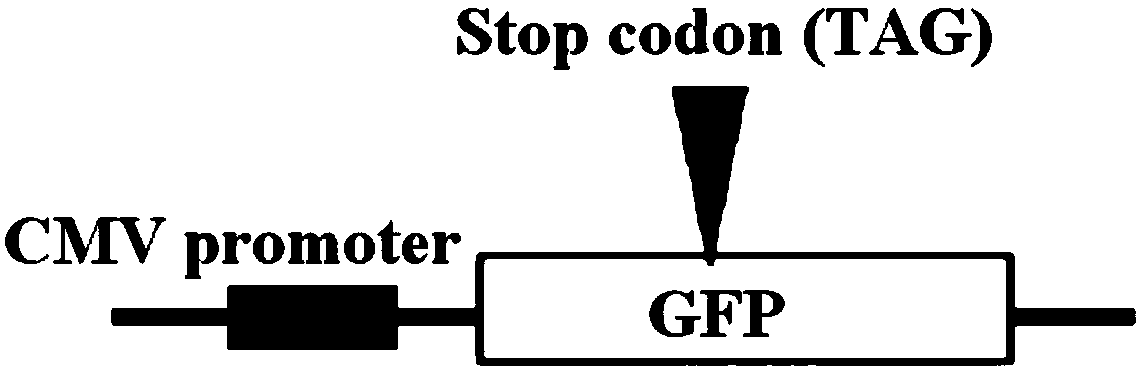
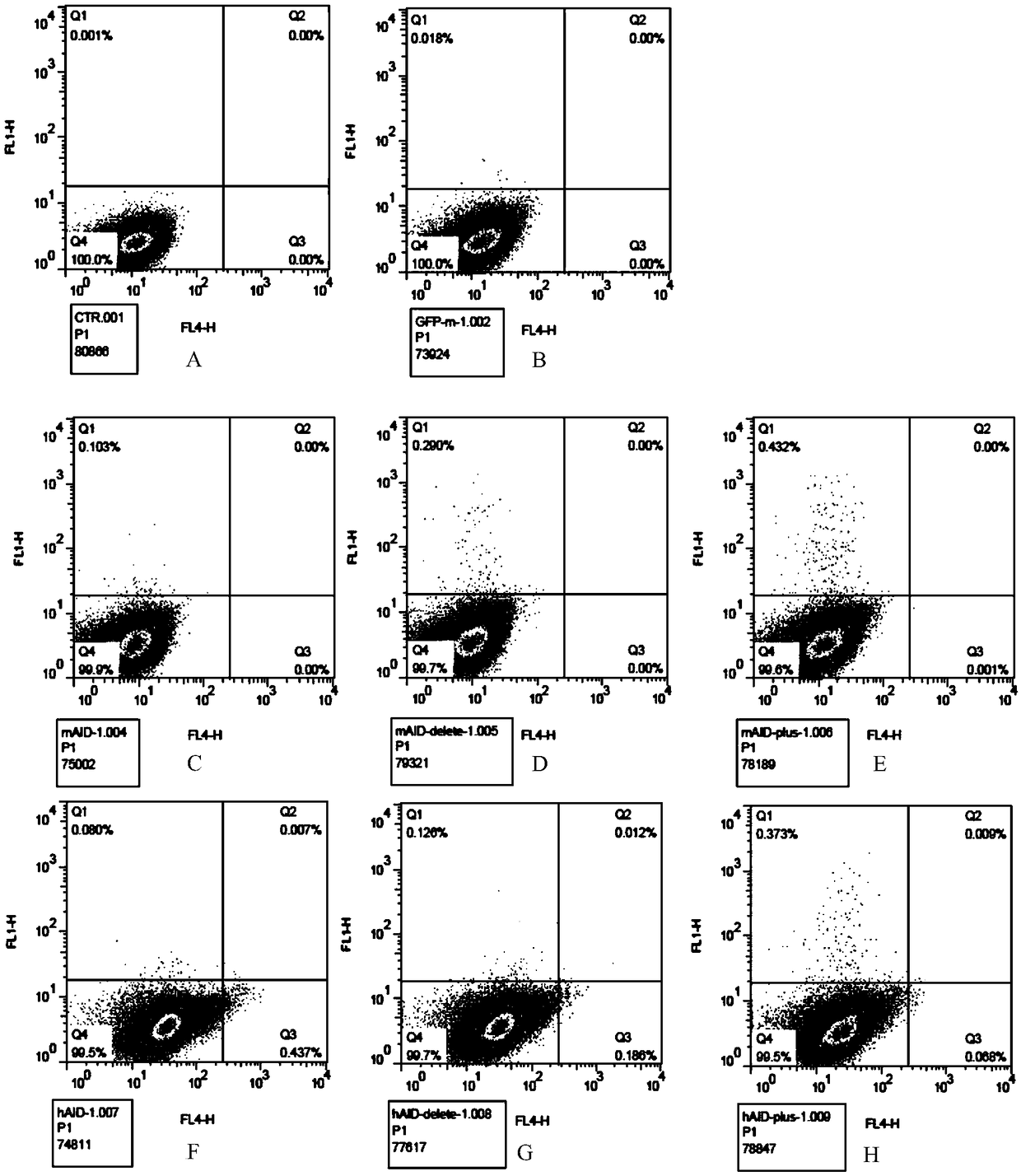
[0051] Embodiment 2 Utilizes GFP reporter gene to detect AID mutation efficiency
[0052] The pCDNA3.1(+)-GFPm plasmid was constructed to detect the mutation ability of the non-integrated AID plasmid. The GFP gene of this plasmid contains a stop codon (TAG), GFPm cannot express the complete GFP protein, and normal cells transfected with this plasmid will not produce green fluorescence (see figure 2 ). If the plasmid and the non-integrated AID plasmid are co-transfected into CHO cells, the TAG stop codon in part of the GFPm gene is mutated into a codon encoding an amino acid, thereby expressing the complete GFP protein and making the cell produce green fluorescence. The ratio of fluorescent cells detected by the instrument can indirectly detect the mutation ability of AID.
[0053] Experimental steps:
[0054] 1. Cell culture
[0055] The selected cells of the present invention are CHO cells, cultured using IMDM medium (Hyclone), and its formula is basic medium with 10% fe...
Embodiment 3
[0069] Example 3 Affinity Maturation Evolution of TNF-α Antibody in CHO Cells Using AID Mutants
[0070] 1. Cell culture
[0071] The cells used in this example are CHO-TNF-α cells, which were stored in our laboratory. The Puro fragments present in CHO cells were replaced with anti-TNF-α fragments, and the cells capable of stably expressing anti-TNF-α antibodies were obtained. cells, named CHO-TNF-α. This example is based on CHO-TNF-α cells to mutate antibody fragments and build a library.
[0072] CHO-TNF-α cells that can stably express anti-TNF-α antibodies based on CHO (Chinese hamster ovary cells) cells are cultured in IMDM medium (Hyclone), and its formula is basic medium supplemented with 10% fetal bovine serum (fatal bovine serum, FBS, Hyclone), 100×HT, 4‰ double-antibody (penicillin and streptomycin) with a final concentration of 100U / mL, at 37°C containing 5% CO 2 cultured in a humidified incubator.
[0073] 2. Cell transfection
[0074] The basic process of lipo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com