Application of abietane type diterpene compounds
A compound and diterpenoid technology, applied in the field of rosinane-type diterpenoids, to achieve the effect of wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of scrodentoid A
[0039] a) Take Scrophulariaceae serrata as raw material, add 8 times the amount of 95wt% ethanol aqueous solution, reflux extraction 3 times, each time for 2 hours; combine the filtrates, concentrate under reduced pressure to obtain a liquid extract; suspend the obtained liquid extract In water, extract 4 times with dichloromethane; combine the extracts, concentrate under reduced pressure to an extract, and obtain a crude extract;
[0040] b) The crude extract is separated by silica gel column chromatography, gradient elution is carried out with petroleum ether and ethyl acetate according to the volume ratio of 100:0 to 50:50, TLC thin layer analysis, the fractions containing scrodentoid A are collected, and the Concentrate under pressure to obtain the crude product of scrodentoidA;
[0041] c) The crude product of scrodentoid A is separated by MCI column, gradient elution is carried out with methanol and water according to the...
Embodiment 2
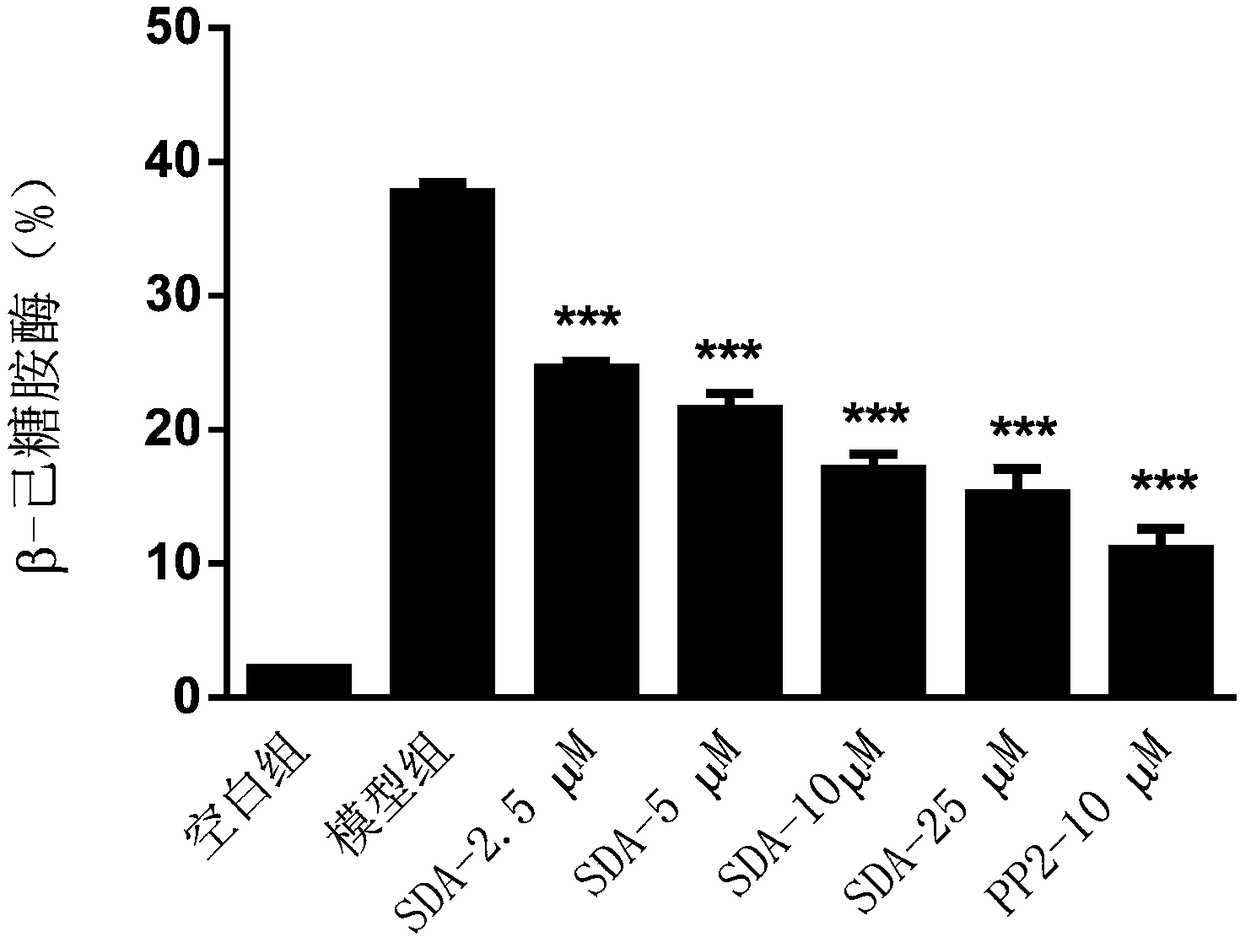
[0046] Example 2: Detecting the release of β-hex in mast cells, and analyzing the impact of scrodentoid A (abbreviated as SDA) on the release of β-hex from mast cells
[0047] Detect the effect of scrodentoid A on IgE-mediated release of β-hex from BMMC cells: use BMMC cells, add anti-mouse-DNP-IgE overnight, after drug intervention, then add DNP-BSA to stimulate BMMC cells to degranulate, detect The release amount of β-hex, all data are expressed as mean±SE, n=3, *P<0.05, **P<0.01, ***P<0.001.
[0048] (1) BMMC cell culture
[0049] Extract the bone marrow of C57BL / 6 mice, use BMMC cell special culture medium at 37°C, 5% CO 2 Four weeks in the incubator.
[0050] (2) Cell sensitization
[0051] Add 0.1 μg / ml anti-mouse-DNP-IgE to BMMC cells, at 37°C, 5% CO 2 Incubate overnight in the incubator.
[0052] (3) Dosing intervention
[0053] After 24 hours, different concentrations (2.5, 5, 10, 25 μM) of scrodentoid A or positive drug (10 μM PP2) were added to intervene for 3...
Embodiment 3
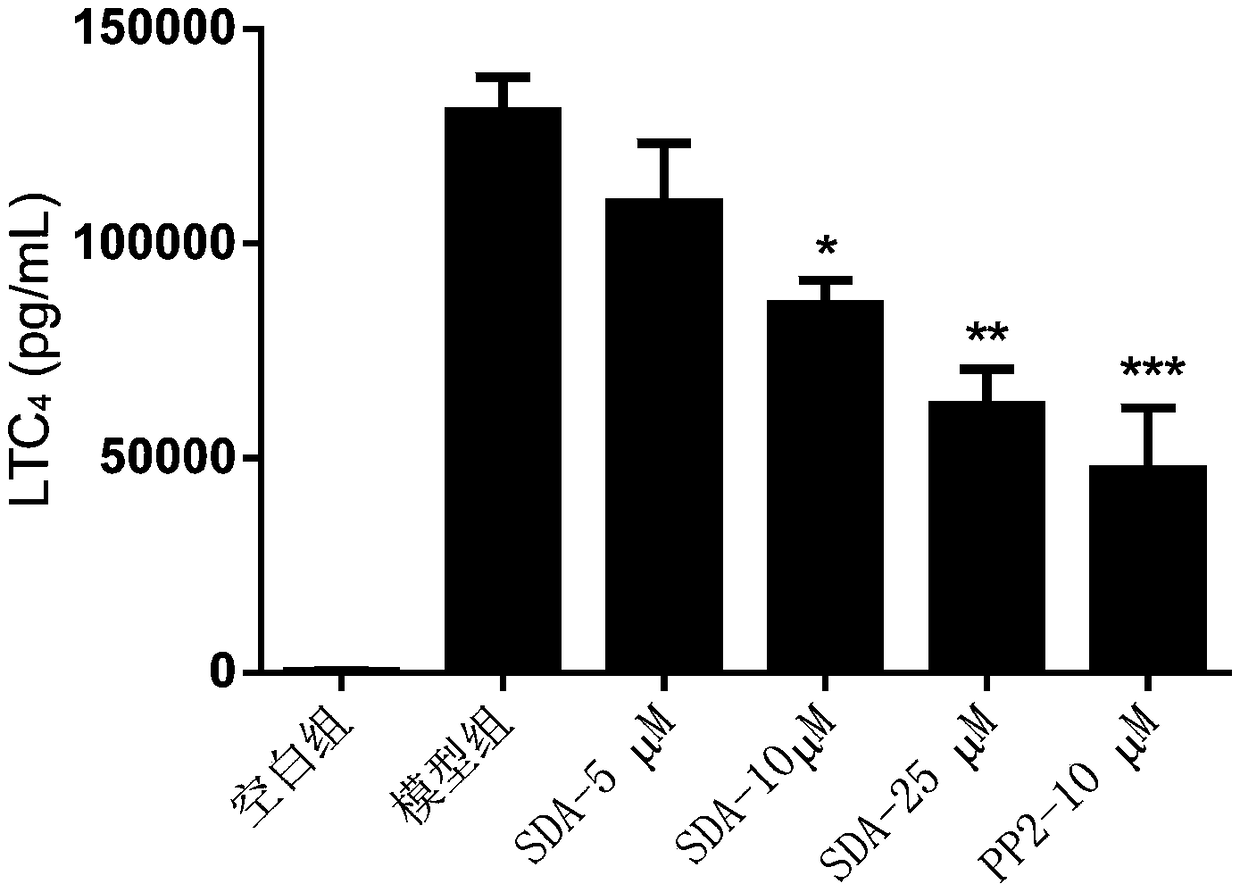
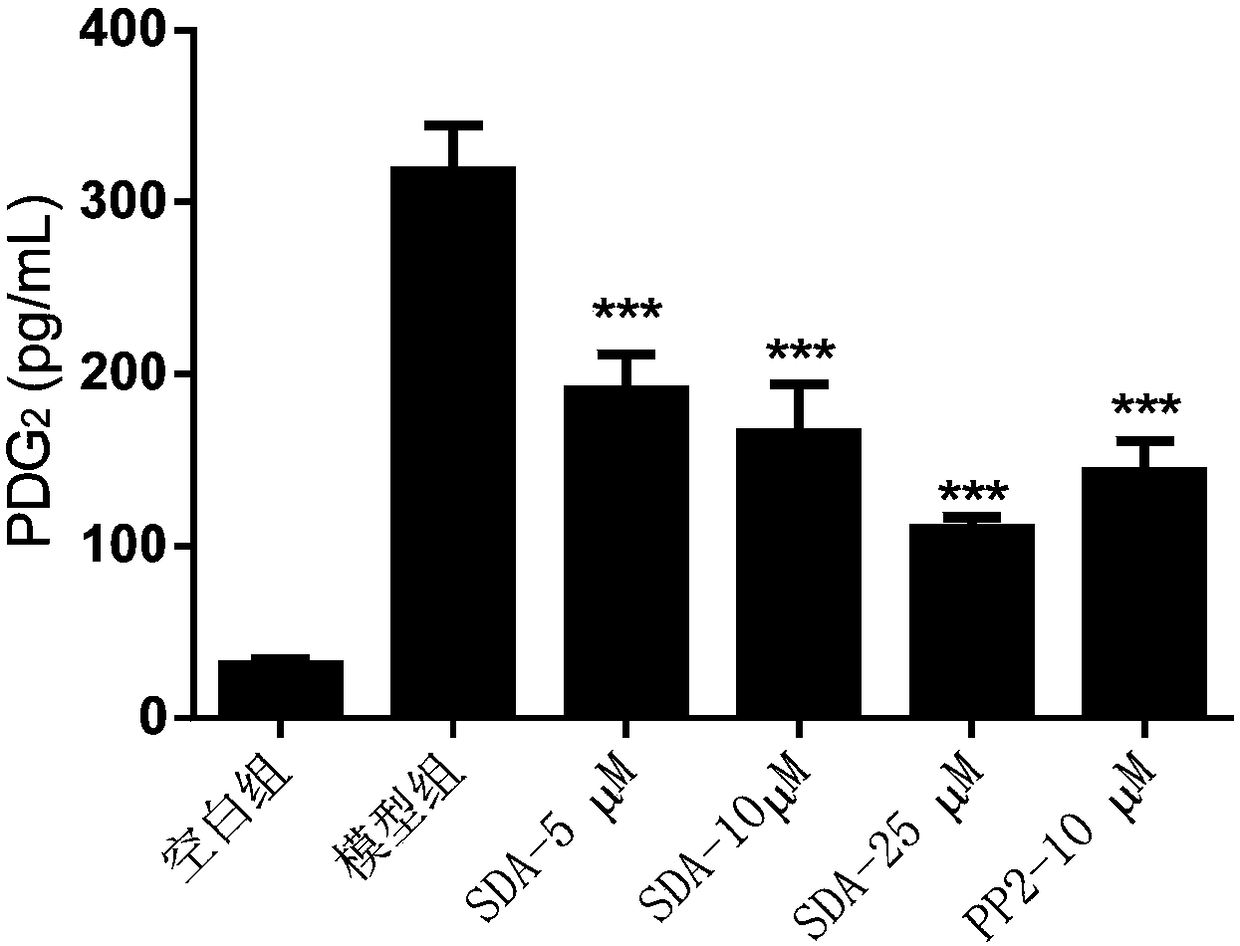
[0062] Example 3: Detecting the release of lipid mediators in mast cells and analyzing the impact of scrodentoid A (abbreviated as SDA) on the release of mast cell lipid mediators
[0063] Detect the effect of scrodentoid A on IgE-mediated release of lipid mediators from BMMC cells: use BMMC cells, add anti-mouse-DNP-IgE overnight, after drug intervention, then add DNP-BSA to stimulate BMMC cells to release lipid mediators, Detect its LTC 4 and PDG 2 All data are expressed as mean±SE, n=3, *P<0.05, **P<0.01, ***P<0.001.
[0064] (1) BMMC cell culture
[0065] Extract the bone marrow of C57BL / 6 mice, use BMMC cell special culture medium at 37°C, 5% CO 2 Cultured in the incubator for four weeks, the mast cell purity was detected to be higher than 99%.
[0066] (2) Cell sensitization
[0067] Add 0.1 μg / ml anti-mouse-DNP-IgE to BMMC cells, at 37°C, 5% CO2 Incubate overnight in the incubator.
[0068] (3) Dosing intervention
[0069] After 24 hours, different concentrations...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com