Aflatoxin B1 control compound biological agent and application thereof
A technology of aflatoxin and biological preparations, applied in the field of feed, can solve problems affecting animal safety and palatability, poor practicality of physical detoxification, and inability to meet production needs, so as to enhance the body's immunity, reduce use, and maintain intestinal tract Healthy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
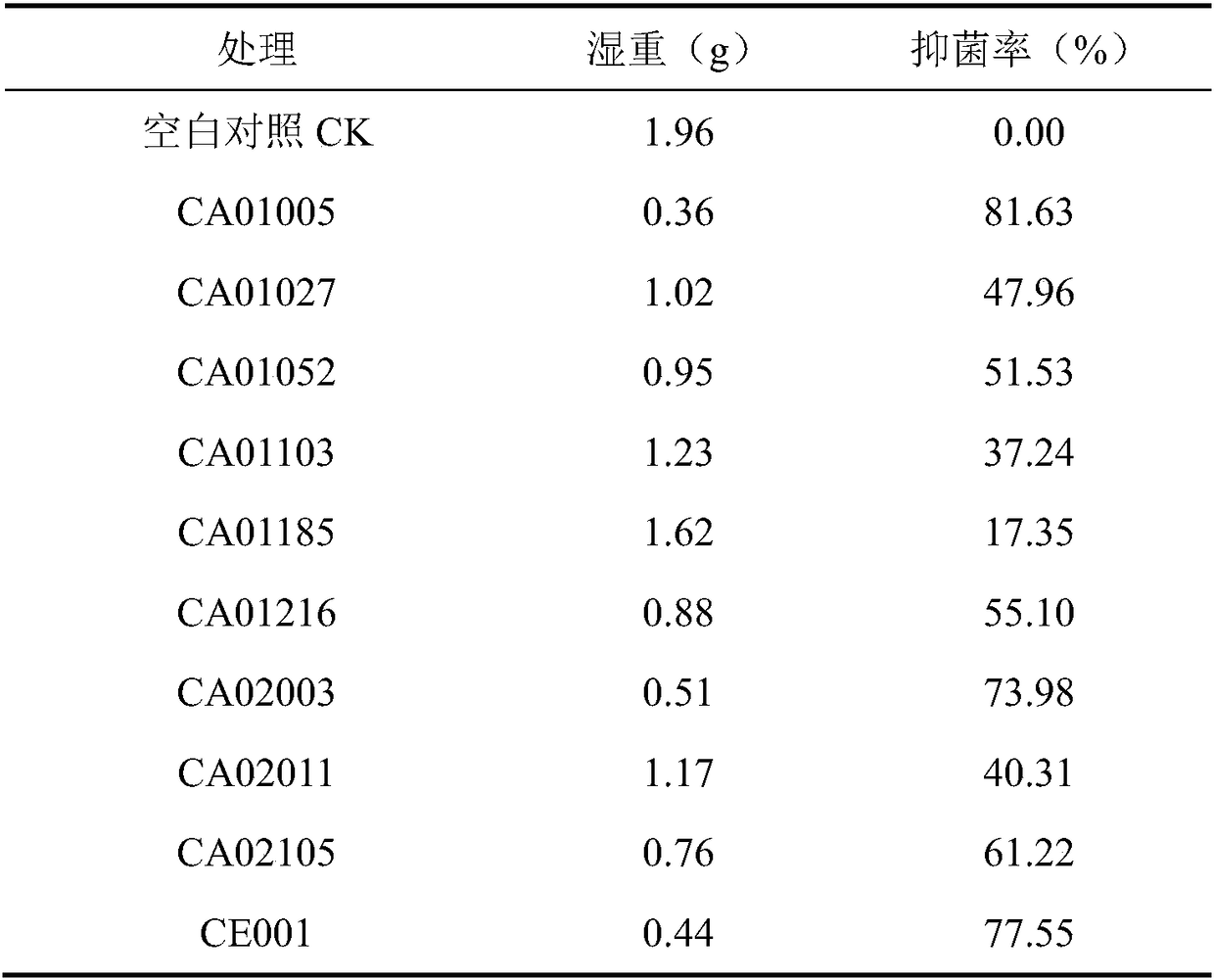
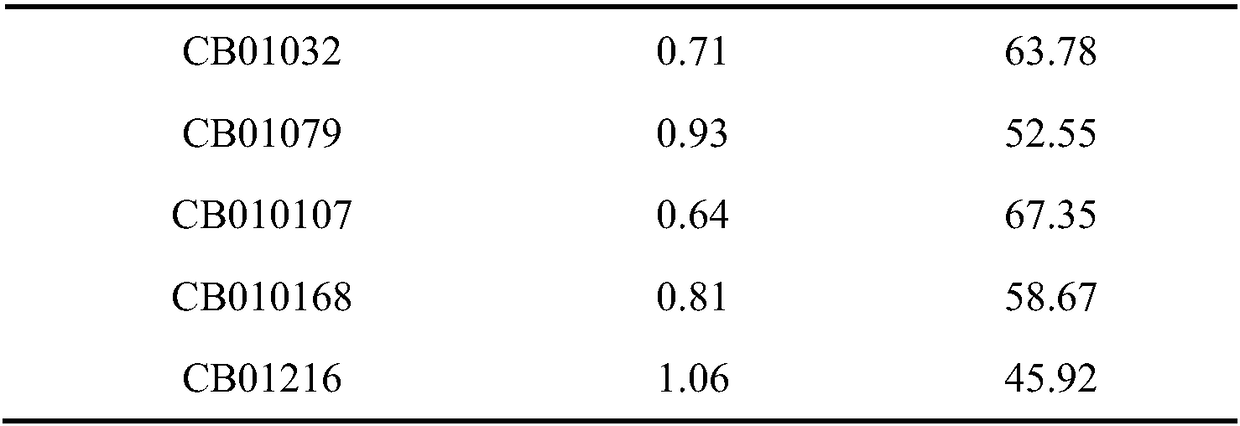
[0033] Example 1 Screening and identification of antagonistic strains of Aspergillus flavus and bacterial strains degrading aflatoxin B1
[0034] 1 Material method
[0035] 1.1 Test material
[0036] 1.1.1 Reagents
[0037] Coumarin, Aflatoxin B 1 , Aflatoxin B 1 Detection kit.
[0038] 1.1.2 Medium
[0039] (1) Potato dextrose agar medium
[0040] Potato dextrose agar medium 38g, distilled water (ddH 2 O) 1000mL, sterilize at 121°C for 30min.
[0041] (2) Potato Dextrose Broth Liquid Medium
[0042] Potato dextrose broth medium 35g, distilled water (ddH 2 O) 1000mL, autoclave at 121°C for 30min.
[0043] (3) Liquid activated medium
[0044] Peptone 10g, yeast powder 5g, sodium chloride 10g, distilled water (ddH 2 O) 1000mL, autoclave at 121°C for 30min.
[0045] (4) Primary screening culture medium
[0046] Potassium dihydrogen phosphate 0.25g, magnesium sulfate heptahydrate 0.25g, potassium nitrate 0.50g, ammonium sulfate 0.50g, calcium chloride 0.005g, ferric ch...
Embodiment 2
[0078] Embodiment 2 controls aflatoxin B 1 Preparation of complex biologics
[0079] 1. the production method of bacillus subtilis or bacillus licheniformis comprises the steps:
[0080] (1) Inoculate Bacillus subtilis or Bacillus licheniformis on the slant medium, and culture at 30-40° C. for 12-24 hours.
[0081] (2) Inoculate the Bacillus subtilis or Bacillus licheniformis colony cultivated on the slant in the seed medium, the liquid filling volume is 50-150mL / 500mL, and cultivate 12- 24 hours.
[0082] (3) Inoculate the seed solution of Bacillus subtilis or Bacillus licheniformis in the seed tank with a weight ratio of 0.1-0.5%, and the filling volume is 50-150L / 500L, at 30-40°C and 120-180 rpm Incubate for 12-24 hours.
[0083] (4) Inoculate the seed tank culture solution in the fermenter with a weight ratio of 0.1-0.5%, the tank pressure is 0.01-0.05MPa, the stirring speed is 120-180 rpm, and the ventilation ratio is 0.5-1:0.1-0.5( volume ratio), the fermentation ti...
Embodiment 3
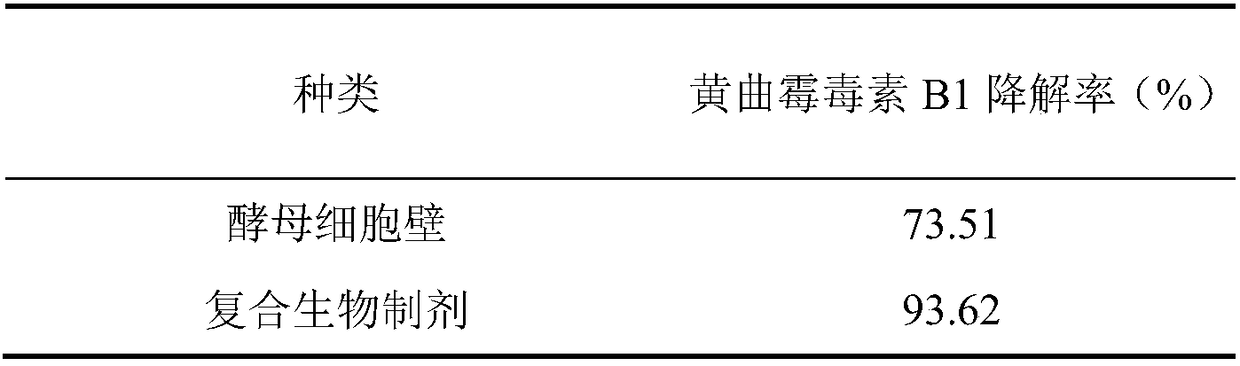
[0094] Embodiment 3 Aflatoxin B 1 detox effect
[0095] Adding different detoxification products to moldy feed to compare aflatoxin B 1 As for the detoxification effect, it can be seen from Table 2 that the degradation rate of aflatoxin B1 by the composite biological preparation is significantly higher than that of the yeast cell wall.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com