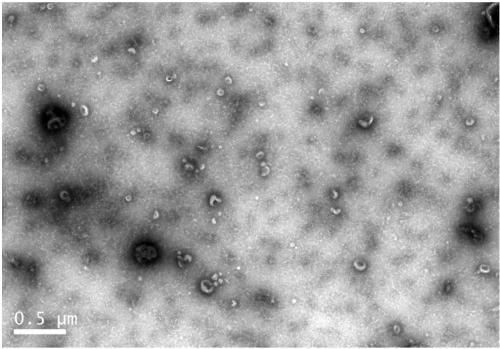
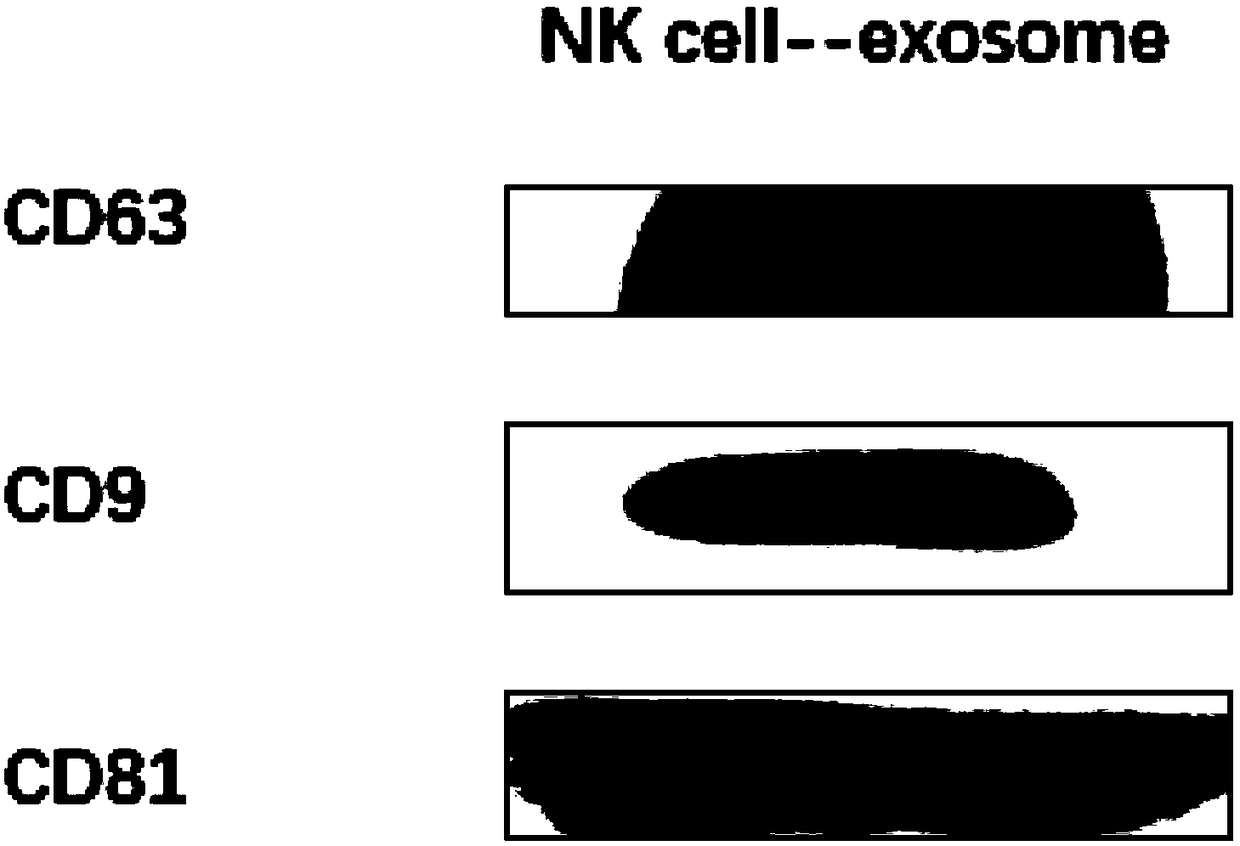
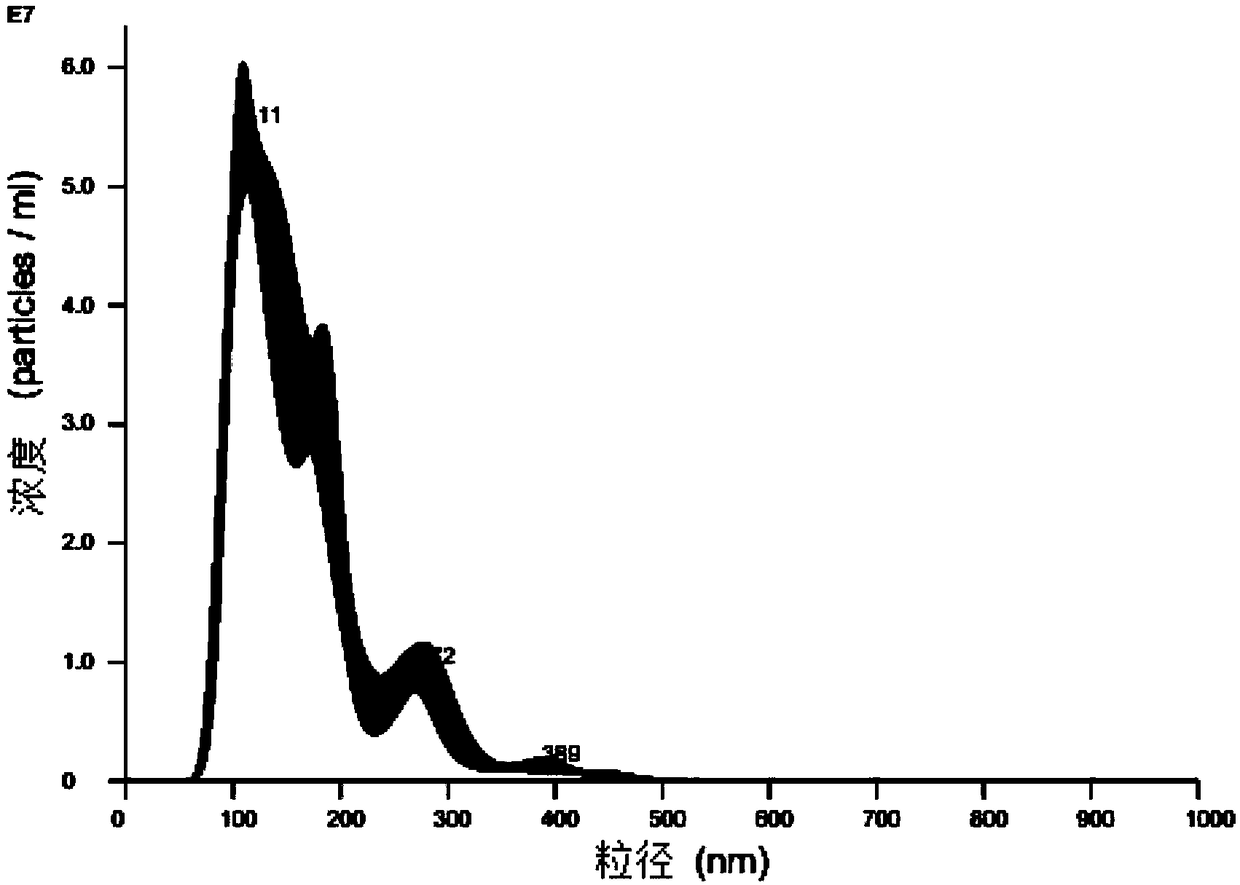
Controllable large-scale preparation method of NK cell exosomes
A technology of NK cells and phosphate buffer solution, which is applied in the field of controllable and large-scale preparation of NK cell exosomes, can solve the problems of poor removal of impurity proteins and low yield, and achieve high scientific research value and uniform particle size distribution , the effect of high safety factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Dilute the collected NK cell culture supernatant with phosphate buffered saline at 4°C, centrifuge at 3,000 rpm for 8 min, precipitate into cells, remove the precipitate, and keep the supernatant;
[0028] (2) Centrifuge the supernatant obtained in step (1) at 9,000 rpm for 15 minutes at 4°C, retain the supernatant, and discard the precipitate;
[0029] (3) Dilute the supernatant obtained in step (2) with phosphate buffer at 4°C, continue to centrifuge at 3,500 rpm for 5 min with a 110kDa ultrafiltration tube to remove small molecular impurities and concentrate the cell supernatant , to reduce the workload and cost of the subsequent ultra-high-speed centrifugation;
[0030] (4) Centrifuge the concentrated supernatant obtained in step (3) at a speed of 100,000 g at 4°C for 60 min. After the centrifugation, discard the supernatant and retain the precipitate;
[0031] (5) Resuspend the precipitate obtained in step (4) with phosphate buffer and transfer it to a dialysi...
Embodiment 2
[0037] (1) Dilute the collected NK cell culture supernatant with phosphate buffered saline at 4°C, centrifuge at 3,000rpm for 9min, precipitate into cells, remove the precipitate, and keep the supernatant,
[0038] (2) Centrifuge the supernatant obtained in step (1) at a speed of 10,000 rpm for 20 minutes at 4°C, retain the supernatant, and discard the precipitate;
[0039] (3) Dilute the supernatant obtained in step (2) with phosphate buffer at 4°C, continue to centrifuge at 3,500 rpm for 5 min with a 110kDa ultrafiltration tube to remove small molecular impurities and concentrate the cell supernatant , to reduce the workload and cost of the subsequent ultra-high-speed centrifugation.
[0040] (4) The concentrated supernatant obtained in step (3) was centrifuged at 100,000 g for 60 min at 4°C. After centrifugation, the supernatant was discarded and the precipitate was retained.
[0041] (5) The precipitate obtained in step (4) was resuspended in phosphate buffer and transfer...
Embodiment 3
[0043] (1) Dilute the collected NK cell culture supernatant with phosphate buffered saline at 4°C, centrifuge at 3,000rpm for 8min, precipitate into cells, remove the precipitate, and keep the supernatant,
[0044] (2) Centrifuge the supernatant obtained in step (1) at 10,000 rpm for 10 min at 4°C, retain the supernatant, and discard the precipitate;
[0045] (3) Dilute the supernatant obtained in step (2) with phosphate buffer at 4°C, continue centrifuging at 3,000 rpm for 10 min with a 110kDa ultrafiltration tube to remove small molecule impurities and concentrate the cell supernatant , to reduce the workload and cost of the subsequent ultra-high-speed centrifugation.
[0046] (4) The concentrated supernatant obtained in step (3) was centrifuged at 100,000 g for 60 min at 4°C. After centrifugation, the supernatant was discarded and the precipitate was retained.
[0047] (5) The precipitate obtained in step (4) was resuspended in phosphate buffer and transferred to a dialysi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com