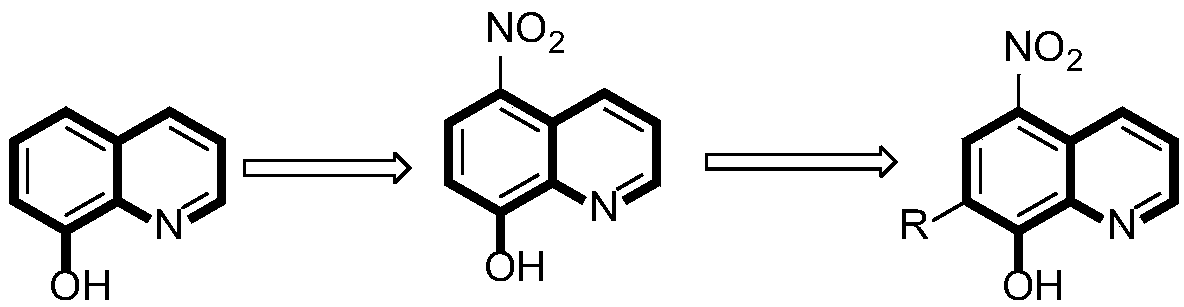
8-hydroxyquinoline compound as well as preparation method and application thereof in preventing and treating agricultural disease
A technology of hydroxyquinoline and compounds, applied in the field of 8-hydroxyquinoline compounds and their preparation and application in the prevention and control of agricultural diseases, which can solve the problems of poor control effect, difficulty in disease control, increased and limited control costs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
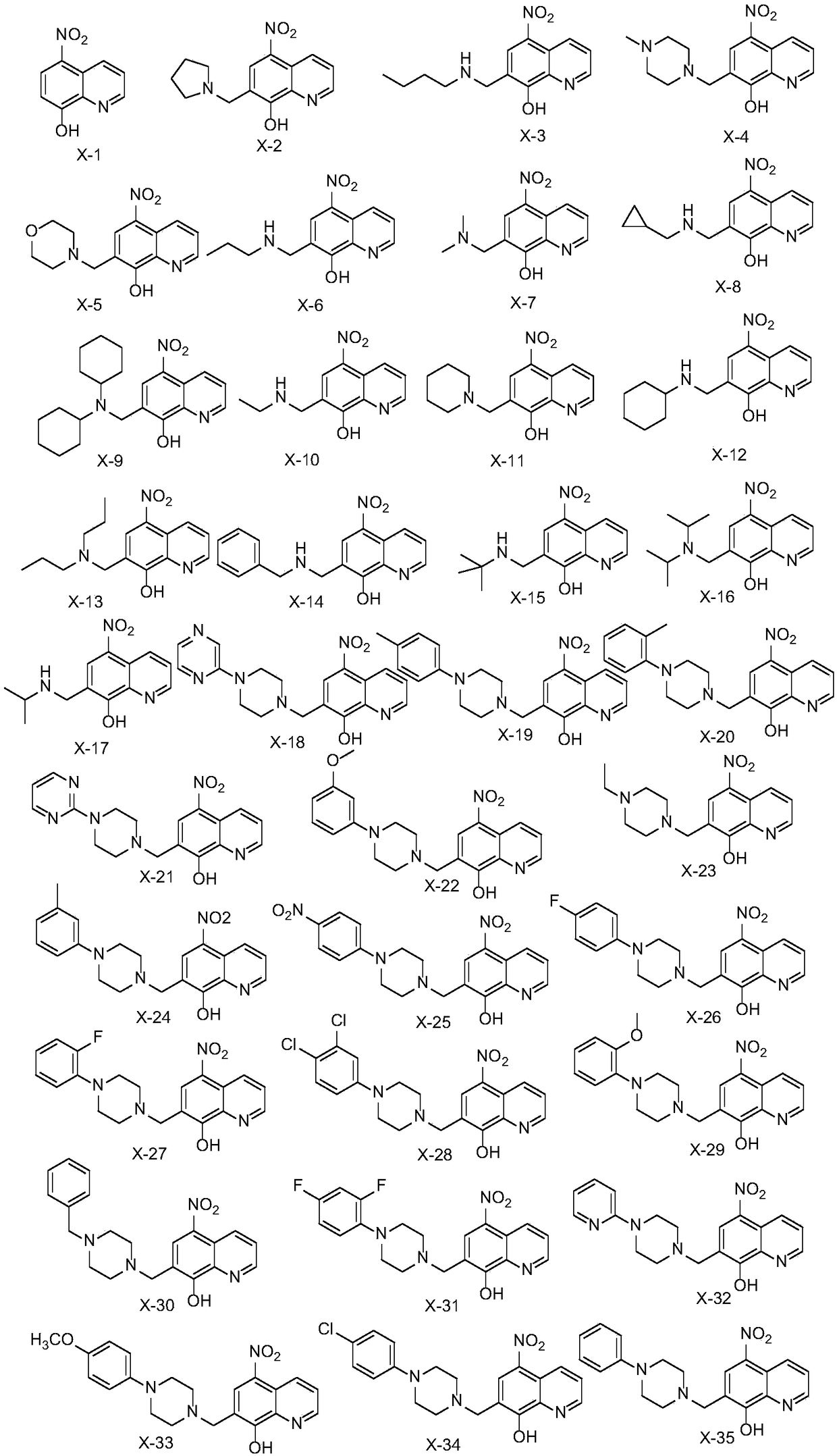
[0011] Synthesis of Quinoline Derivatives (X-2~X-35)
[0012]
[0013] Its concrete synthetic operation is as follows:
[0014] 5-nitro-8 hydroxyquinoline was prepared according to the method reported in the literature. And 0.5g (2.63mmol) 5-nitro-8 hydroxyquinoline and 0.32mL formaldehyde were dissolved in 20ml absolute ethanol. To this solution was added 2 equivalents of the corresponding amine. The mixture was refluxed at 80°C for 24h. The reaction solution was filtered to obtain a precipitate, which was then dissolved in EtOH:H 2 O=1:1 solution was recrystallized to obtain the corresponding product.
Embodiment 2
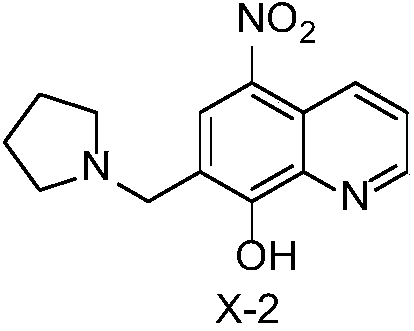
[0016] The synthesis of X-2: experimental procedure is with embodiment 1.
[0017]
[0018] X-2 yellow-green solid, yield 80%; 1 H NMR (400MHz, DMSO-d 6 )δ9.33(dd,J=8.7,1.6Hz,1H), 8.61(d,J=4.0Hz,1H),8.59(s,1H),7.56(dd,J=8.7,4.1Hz,1H), 4.17(s,2H),3.11(s,4H),1.73(t,J=5.9Hz,4H). 13 C NMR (101MHz, DMSO-d 6 )δ158.94,148.08,136.65,135.17,132.68, 132.43,126.47,124.07,113.65,53.73,53.73,51.59,22.95,22.95.ESI-MS m / z:274.29[M+1] + .
Embodiment 3
[0020] The synthesis of X-3: experimental procedure is with embodiment 1.
[0021]
[0022] X-3 yellow solid, yield 90%; 1 H NMR (400MHz, DMSO-d 6 )δ9.35(d, J=8.7Hz, 1H), 8.61(s, 1H), 8.55(s, 1H), 7.55(dd, J=8.7, 4.1Hz, 1H), 4.59(s, 1H), 4.08(s,2H),2.94(s,2H),1.64(t,J=8.1Hz,2H),1.34(q,J=7.6Hz,2H),0.89(t,J=7.4Hz,3H). 13 C NMR (101MHz, DMSO-d 6 )δ 158.78,148.47,136.65,134.77,132.74,131.96,126.34,123.69,115.71,47.05,44.79,27.91, 19.72,13.96.MS-ESI m / z:276.31[M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com