Bio-catalytic method for synthesizing optical activity 2R-fluorocarboxylic acid and 2R-hydroxy carboxylic acid
A fluorinated carboxylic acid and biocatalysis technology, applied in the field of bioengineering, can solve the problems of harsh reaction conditions, low reaction efficiency, expensive reagents, etc., and achieve the effects of mild reaction conditions, simple process route, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
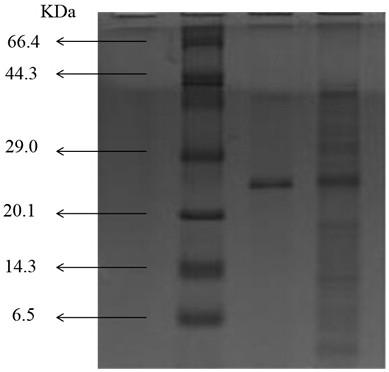
[0035] In this example, optically active R-fluoromandelic acid and R-mandelic acid were synthesized by biocatalysis, as shown in (II).
[0036]
[0037] (Ⅱ)
[0038] In this example, 2-fluoromandelic acid was used as a substrate, and fluoroacetic acid dehalogenase was used as a biocatalyst for chiral resolution to obtain chirally pure 2R-fluoromandelic acid and R-mandelic acid, including the following step:
[0039] (1) Transfer the plasmid expressing fluoroacetic acid dehalogenase into the expression strain, and cultivate and express it.
[0040] Transform the expression plasmid containing fluoroacetic acid dehalogenase gene into Escherichia coli expression strain BL21 (DE3), spread the bacterial solution on the solid medium containing kanamycin, cultivate overnight, and select a single colony containing 50 μg mL -1 Incubate overnight at 37°C in kanamycin lysate broth. Transfer about 1% of the seed culture to super broth, add 50 μg mL -1 Kanamycin. The expression cond...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com