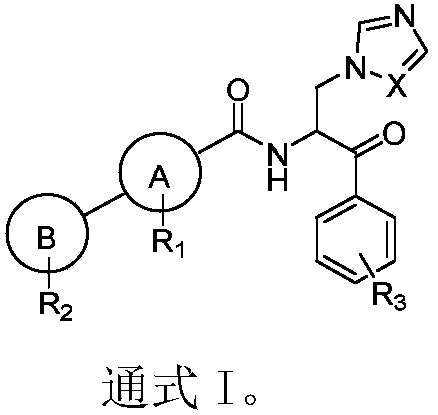
Beta-azoles-phenyl ketone derivative and application thereof
A phenyl, biphenyl technology, applied in hydrates, β-azoles-phenyl ketone derivatives and their pharmaceutically acceptable salts, in the field of medicine for various diseases, can solve liver and kidney toxicity, drug- Drug interactions and drug resistance, narrow antibacterial spectrum, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
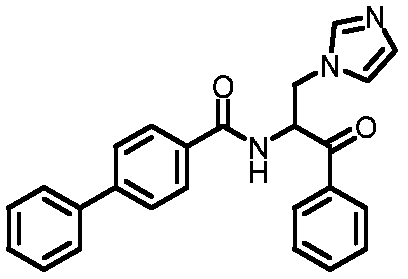
[0091] Example 1: 1-(phenyl)-2-[(1,1'-biphenyl)-4-carboxamido]-3-(1H-imidazol-1-yl)-acetone
[0092]
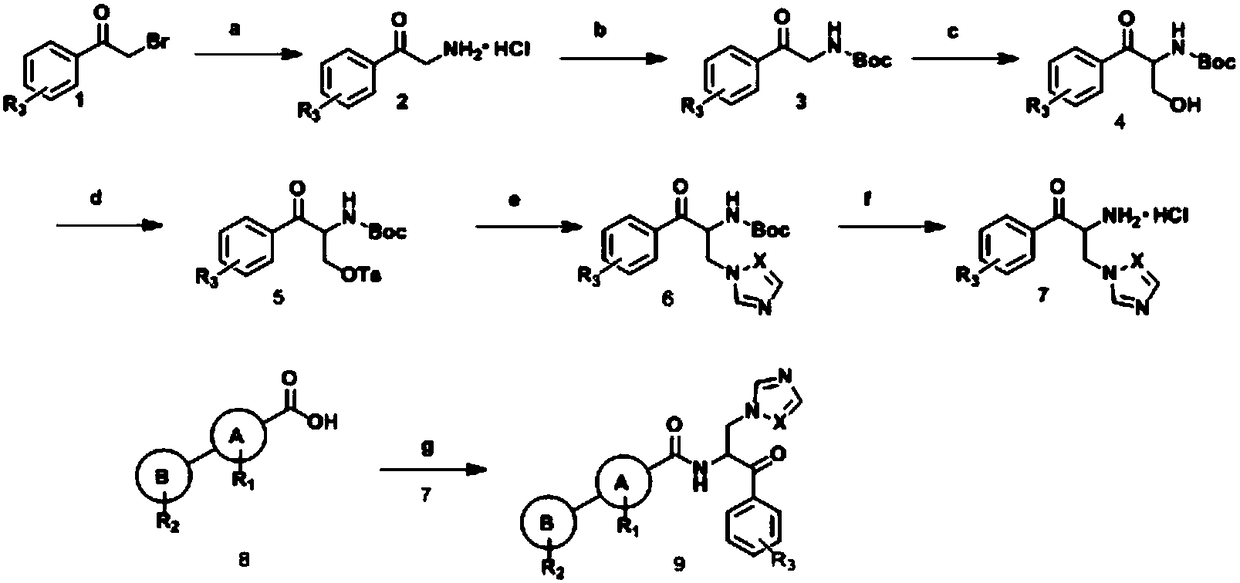
[0093] The synthetic route is as follows:
[0094]
[0095] Reagents and conditions: (a) i) urotropine, chloroform; ii) 37% hydrochloric acid, ethanol; (b) (Boc) 2 O, sodium bicarbonate, methanol; (c) sodium bicarbonate, formaldehyde (aq), methanol; (d) TsCl, TEA, DMAP, DCM; (e) imidazole, K 2 CO 3 , DMF; (f) hydrochloric acid-ethanol; (g) tetrakistriphenylphosphopalladium, potassium carbonate, dioxane: water; (h) HOBt, EDCI, DIEA, DMF.
[0096] Add 2.0g (10.05mmol) of α-bromoacetophenone and 1.4g (10.05mmol) of urotropine into 60mL of chloroform in turn, react at 50 degrees Celsius, TLC detects that the reaction is complete, filter, filter cake, 5mL concentrated Add hydrochloric acid to 50mL ethanol, reflux for 4h, filter, and concentrate the filtrate to prepare intermediate 1-2.
[0097] Intermediate 1-2 (10.05mmol), (Boc) 2 O (15mmol) and sodium bicarbonate (22m...
Embodiment 2
[0105] Example 2: 1-(2-fluoro-phenyl)-2-[(1,1'-biphenyl)-4-carboxamido]-3-(1H-imidazol-1-yl)-acetone
[0106]
[0107] ESI-MS[M+H] + (m / z): 414.1.
[0108] 1 H NMR(600MHz,DMSO)δ9.25(d,J=7.7Hz,1H),7.81-7.78(m,1H),7.77-7.71(m,4H),7.70–7.67(m,2H),7.64– 7.58(m,2H),7.48(t,J=7.7Hz,2H),7.40(t,J=7.4Hz,1H),7.33-7.29(m,1H),7.18(s,1H),6.86(s , 1H), 5.39–5.28 (m, 1H), 4.67 (dd, J=14.2, 4.2Hz, 1H), 4.42 (dd, J=14.2, 9.5Hz, 1H).
Embodiment 3
[0109] Example 3: 1-(3-Fluoro-phenyl)-2-[(1,1'-biphenyl)-4-carboxamido]-3-(1H-imidazol-1-yl)-propanone.
[0110]
[0111] ESI-MS[M+H] + (m / z): 414.1.
[0112] 1 H NMR (600MHz, DMSO) δ9.21(d, J=8.3Hz, 1H), 7.86(d, J=7.8Hz, 1H), 7.80(d, J=8.5Hz, 2H), 7.78–7.76(m ,1H),7.74(d,J=8.5Hz,2H),7.72–7.68(m,2H),7.62(s,1H),7.59–7.55(m,1H),7.50–7.47(m,3H), 7.40(t, J=7.4Hz, 1H), 7.22(s, 1H), 6.84(s, 1H), 5.72–5.69(m, 1H), 4.60(dd, J=14.1, 4.5Hz, 1H), 4.44 (dd, J = 14.1, 9.6 Hz, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com