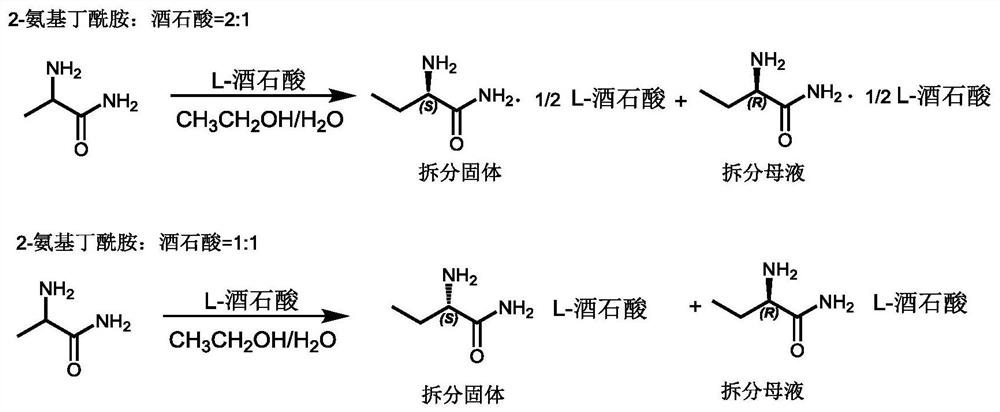
A kind of resolution method of (s)-(+)-2-aminobutanamide hydrochloride
A technology of aminobutyramide and hydrochloride, which is applied in the direction of organic chemical methods, chemical instruments and methods, and the preparation of carboxylic acid amide optical isomers, can solve the problem of low resolution yield, and achieve simple and efficient operation of each step. The effect of reducing environmental pollution and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
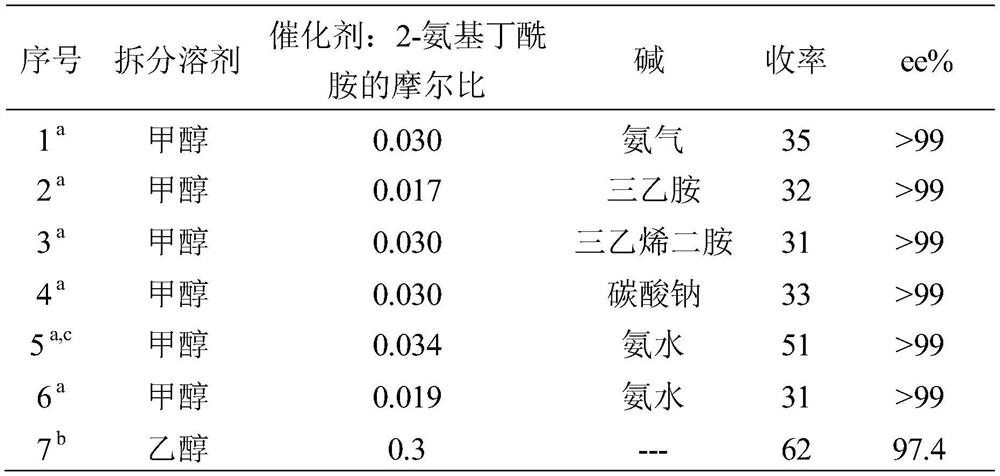
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 The preparation of optically pure (S)-(+)-2 aminobutanamide hydrochloride
[0031] Take by weighing (S)-(+)-2-aminobutyramide resolution mother liquor 5kg, its main component is the L-tartrate of (R)-(-)-2-aminobutanamide, and resolution solvent methanol, through Quantitatively it contains about 250 g of (R)-(-)-2-aminobutyramide.
[0032] Most of the methanol was removed under reduced pressure distillation at 60°C, 1.5kg of water was added, and ammonia gas was introduced to saturation, then 7.89g of salicylaldehyde was added, heated to 90°C, and reacted for about 8 hours. Stop heating, cool to room temperature, concentrate under reduced pressure to remove ammonia water, adjust the pH to about 7 with 2mol / L hydrochloric acid, add about 2.5L of methanol, cool to 15°C, a large amount of white solid precipitates, filter, and drain to obtain pure The product (S)-(+)-2-aminobutyramide tartrate is about 302g, and its optical purity is >99%.
[0033] Add about 3...
Embodiment 2
[0034] Embodiment 2 Preparation of optically pure (S)-(+)-2 aminobutyramide hydrochloride
[0035] Take (S)-(+)-2-aminobutyramide and resolve 3.5kg of mother liquor, its main components are (R)-(-)-2-aminobutanamide, L-tartaric acid, and resolution solvent methanol, after Quantitatively it contains about 175 g of (R)-(-)-2-aminobutyramide.
[0036] Remove most of the methanol under reduced pressure distillation at 60°C, add 1.0kg of water, add triethylamine until the pH of the system reaches 8.5, then add 2.17g of n-butyraldehyde, heat to 80°C, and react for about 6 hours. Stop heating, cool to room temperature, concentrate under reduced pressure to remove excess triethylamine, adjust the pH to about 7 with 2mol / L hydrochloric acid, add about 2L of methanol, cool to 15°C, a large amount of white solid precipitates, filter, and drain , to obtain about 200 g of pure (S)-(+)-2-aminobutyramide tartrate.
[0037] Add about 200g of the above pure (S)-(+)-2-aminobutyramide tartrate...
Embodiment 3
[0038] Embodiment 3 Preparation of optically pure (S)-(+)-2 aminobutanamide hydrochloride
[0039] Weigh 5kg of (S)-(+)-2-aminobutanamide resolution mother liquor, its main components are (R)-(-)-2-aminobutanamide, L-tartaric acid, and resolution solvent ethanol, after quantitative It contains about 250 g of (R)-(-)-2-aminobutanamide.
[0040] Remove most of the methanol under reduced pressure distillation at 60°C, add 1.5kg of water, 482g of triethylenediamine, and 7.89g of salicylaldehyde, heat to 85°C, and react for about 5 hours. Stop heating, cool to room temperature, concentrate under reduced pressure to remove part of the water, adjust the pH to about 7 with 1mol / L hydrochloric acid, add about 2.5L of methanol, cool to 15°C, a large amount of white solid precipitates, filter, and drain to obtain The pure product (S)-(+)-2-aminobutyramide tartrate is about 298g, and its optical purity is >99%.
[0041] Add about 298g of the above pure (S)-(+)-2-aminobutyramide tartrate t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com