Method for preparing anti-freezing type 1,3-dihydroxy methyl-5,5-dimethylheine DMDMH
A technology of dimethylhydantoin and dimethylol, which is applied in the field of preparation of daily chemical additives, can solve problems such as strong odor, high free formaldehyde, and strong irritation, and achieves simple and convenient preparation process, simple reaction process, and free The effect of low aldehyde content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 (example of the present invention)
[0027] A 2L three-neck round bottom flask is equipped with a stirrer and a temperature regulator. Put 600g of formaldehyde into the flask, and adjust the pH to 7.4 with aqueous sodium hydroxide solution. 500g of hydantoin was added to the flask, and a violent exotherm was observed, the temperature rose, and most of the hydantoin was dissolved. The pH was adjusted to 8.3 with aqueous sodium hydroxide solution and the reaction mixture was stirred at 55°C for a further 1 hour. 175 g of water were added, then cooled to room temperature. Add 10.5g NH 4 Cl, stir well. Adjust the pH to 7.0.
[0028] The above product is the antifreeze type 1,3 dimethylol-5,5 dimethylhydantoin DMDMH.
Embodiment 2
[0029] Embodiment 2 (comparison of embodiment 1, does not add NH 4 Cl)
[0030] A 2L three-neck round bottom flask is equipped with a stirrer and a temperature regulator. Put 600g of formaldehyde into the flask, and adjust the pH to 7.2 with aqueous sodium hydroxide solution. 500g of hydantoin was added to the flask, and a violent exotherm was observed, the temperature rose, and most of the hydantoin was dissolved. The pH was adjusted to 8.1 with aqueous sodium hydroxide solution and the reaction mixture was stirred at 55°C for a further 1 hour. 175 g of water were added, then cooled to room temperature. Adjust the pH to 6.9.
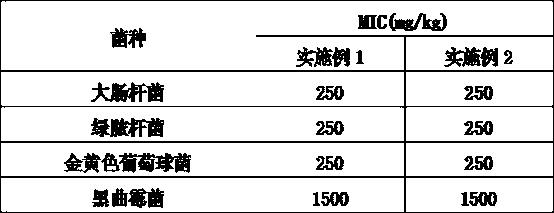
[0031] The antimicrobial effect of embodiment 1 and embodiment 2 product is as shown in table 1. Embodiment 1 (the product of the present invention) is equivalent to the antimicrobial ability of embodiment 2 products.
[0032] Table 1, the minimum inhibitory concentration MIC value of embodiment 1 and embodiment 2 products.
[0033]
[0034] T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com