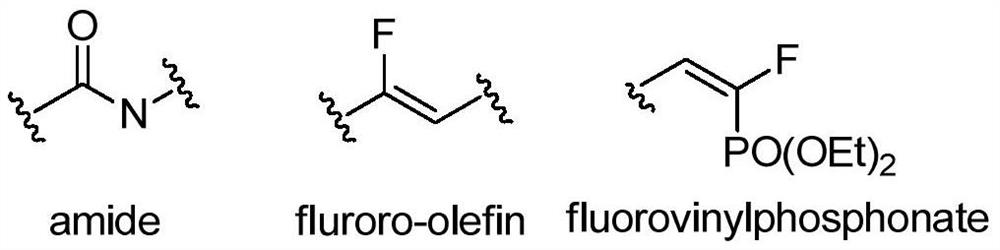
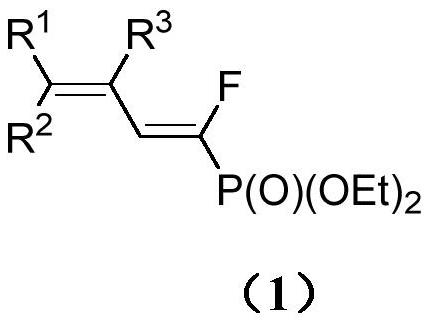
A kind of (e)-3-aryl-1-fluoro-1,3-butadiene phosphonate compound and its synthesis method and application
A technology of butadiene phosphonate and synthesis method, which is applied to compounds of Group 5/15 elements of the periodic table, chemical instruments and methods, organic chemistry, etc., can solve the problem of poor regioselectivity and stereoselectivity and insufficient biological activity Ideal, harsh reaction conditions and other problems, to achieve the effect of high stereoselectivity, high yield and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add 4-methyl-1,1-difluoro-2,3-pentadienephosphonic acid diethyl ester (0.4 mmol), Pd(OAc) to a dry reaction tube 2 (5%mol), PPh 3 (15% mol) aryl boronic acid (0.8mmol), KOH (0.8mmol), 4mL Toluene, placed in a 50 ° C oil bath and stirred, TLC monitoring until the end of the reaction, diluted with ethyl acetate and concentrated by filtration, the crude product with petroleum ether- Ethyl acetate was used as the eluent to separate the product by column chromatography. The reaction process is as follows:
[0036]
[0037] The obtained (E)-3-aryl-1-fluoro-1,3-butadiene phosphonate had the following structure and a yield of 94%.
[0038]
[0039] Diethyl(E)-(1-fluoro-4-methyl-3-phenylpenta-1,3-dien-1-yl)phosphonate
[0040] Yellow oil; IR(film):2927,2850,1454,1377,1270,1115,1018,969,766,708,552cm -1 ; 1 H NMR (400MHz, CDCl 3 ):δ7.33-7.29(m,2H),7.26-7.21(m,1H),7.11-7.09(m,2H),6.76(dd,J H-F =40.4Hz,JH-P =8.8Hz, 1H), 4.18-4.04(m, 4H), 1.95(s, 3H), 1.65(s, 3H), 1.31(...
Embodiment 2
[0042] The operation is the same as before, and the reaction process is as follows
[0043]
[0044] The obtained (E)-3-aryl-1-fluoro-1,3-butadiene phosphonate has the following structure and a yield of 90%.
[0045]
[0046] Diethyl(E)-(1-fluoro-3-(4-methoxyphenyl)-4-methylpenta-1,3-dien-1-yl)phosphonate.
[0047] Yellow oil; IR(film):2966,2918,1609,1512,1454,1377,1260,1115,1027,959,795,533cm -1 ; 1 H NMR (400MHz, CDCl 3 ):δ7.02-6.99(m,2H),6.84-6.81(m,2H),6.72(dd,J H-F =40.4Hz,J H-P =8.4Hz, 1H), 4.17-4.03(m, 4H), 3.77(s, 3H), 1.92(s, 3H), 1.65(s, 3H), 1.30(t, J=7.1Hz, 6H); 13 C NMR (100MHz, CDCl 3 ):δ158.1,147.4(dd,J C-F =282.3Hz,J C-P =234.5Hz), 139.6(d, J C-F =3.0Hz), 132.7, 130.0, 127.5 (d, J C-F =13.6Hz), 122.9(d, J C-F =30.0Hz), 113.2, 62.8(d, J C-P =5.4Hz), 55.0, 22.4, 21.8, 16.2 (d, J C-P = 6.2Hz); 19 F NMR (376MHz, CDCl 3 ):δ-125.9(dd,J F-P =99.6Hz,J H-F =40.2Hz,1F); 31 P NMR (162MHz, CDCl 3 ):δ6.64(d,J F-P =100.1Hz,1P).HRMS Calcd for C 17 h...
Embodiment 3
[0049] The operation is the same as before, and the reaction process is as follows
[0050]
[0051] The obtained (E)-3-aryl-1-fluoro-1,3-butadiene phosphonate has the following formula, and the yield is 84%.
[0052]
[0053] Diethyl(E)-(1-fluoro-3-(4-fluorophenyl)-4-methylpenta-1,3-dien-1-yl)phosphonate.
[0054] Yellow oil; IR(film):2956,2908,1609,1512,1444,1388,1270,1105,1037,814,708,582,552 cm -1 ; 1 H NMR (400MHz, CDCl 3 ):δ7.07-7.03(m,2H),7.01-6.95(m,2H),6.73(dd,J H-F =40.2Hz,J H-P =8.6Hz, 1H), 4.18-4.04(m, 4H), 1.94(s, 3H), 1.63(s, 3H), 1.31(t, J=7.0Hz, 6H); 13 C NMR (100MHz, CDCl 3 ):δ161.5(d,J C-F =243.7Hz), 147.7(dd, J C-F =283.0Hz,J C-P =235.2Hz), 140.4(d, J C-F =3.1Hz), 136.3, 130.5(dd, J C-F =7.9,1.4Hz),127.2(d,J C-F =13.8Hz), 122.3(d, J C-F =30.2Hz), 114.8(d, J C-F =21.2Hz), 62.9(d, J C-P =5.5Hz), 22.4, 21.6, 16.1 (d, J C-F = 6.1Hz); 19 F NMR (376MHz, CDCl 3 ):δ-116.1--116.0(m,1F),-125.5(dd,J F-P =99.3Hz,J H-F =40.2Hz,1F); 31 P NMR (16...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com