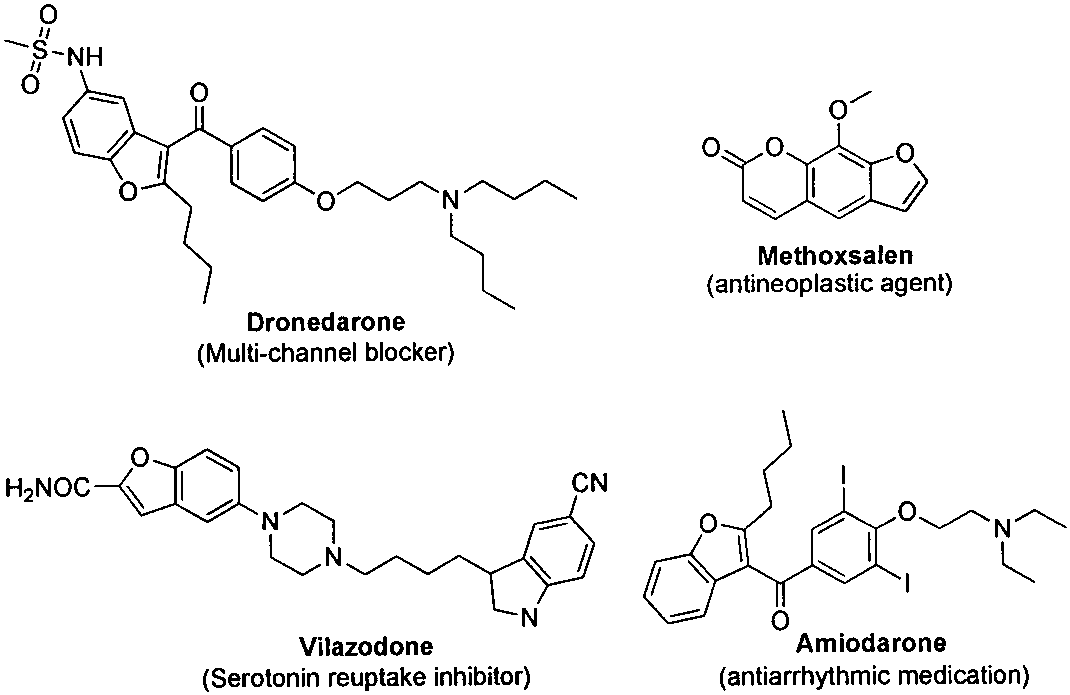
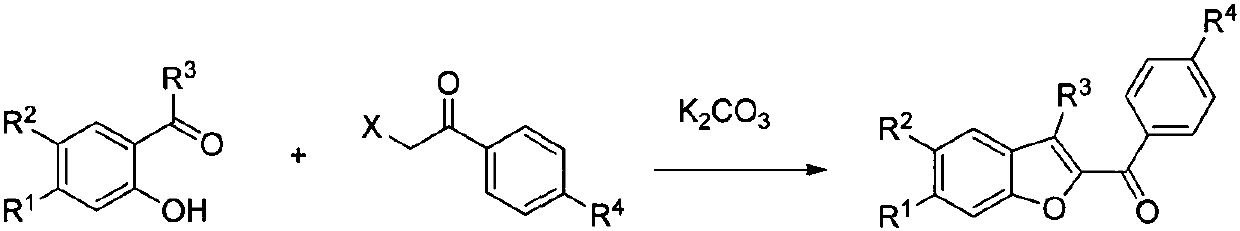
Method for synthesizing benzofuran derivative from phenol and alpha-haloketone
A technology of benzofuran and derivatives is applied in the fields of drug synthesis and chemical product synthesis to achieve the effects of reducing production cost, easy separation and less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the synthesis of benzofuran derivative IIIa
[0029]
[0030] Phenol Ia (2 mmol) and hexafluoroisopropanol (10 mL) were added to a 50 mL two-neck flask equipped with a reflux condenser and under nitrogen protection, and heated to reflux while stirring. After the phenol Ia was completely dissolved, titanium tetrachloride (2mmol) was added, and finally a mixed solution of α-haloketone IIa (2mmol) and hexafluoroisopropanol (2mL) was slowly added dropwise, and the reflux reaction was continued. TLC monitoring. After the reaction was completed, it was quenched by adding saturated aqueous ammonium chloride solution (10 mL), and then extracted with dichloromethane (3×10 mL). The dichloromethane solution obtained by the mixed extraction was concentrated under reduced pressure, and then separated by silica gel column chromatography to obtain the target product IIIa as a yellow oil with a yield of 75%.
[0031] 1 H NMR (600MHz, CDCl 3 )δ7.42 (ddd, J=5.7, 3.3,...
Embodiment 2
[0032] Embodiment 2: the synthesis of benzofuran derivative IIIb
[0033]
[0034] In a 50mL two-necked flask under nitrogen protection, add substituted phenol Ib (2mmol) and trifluoroethanol (15mL), stir at room temperature, after the substituted phenol Ib is completely dissolved, add titanium tetrachloride (3mmol), and finally slowly A mixed solution of α-haloketone IIa (3 mmol) and trifluoroethanol (2 mL) was added dropwise to continue the reaction at room temperature, and the reaction process was monitored by TLC. After the reaction was completed, it was quenched by adding saturated aqueous ammonium chloride solution (10 mL), and then extracted with dichloromethane (3×10 mL). The dichloromethane solution obtained by the mixed extraction was concentrated under reduced pressure, and then separated by silica gel column chromatography to obtain the target product IIIb as a yellow oil with a yield of 80%.
[0035] 1 H NMR (600MHz, CDCl 3)δ7.30(d, J=8.3Hz, 1H), 7.22(s, 1H)...
Embodiment 3
[0036] Embodiment 3: the synthesis of benzofuran derivative IIIc
[0037]
[0038] In a 50 mL two-neck flask equipped with a reflux condenser and under nitrogen protection, add substituted phenol Ic (2 mmol) and trifluoroethanol (10 mL), and heat to reflux while stirring. After the substituted phenol Ic was completely dissolved, titanium tetrachloride (4mmol) was added, and finally a mixed solution of α-haloketone IIa (2mmol) and trifluoroethanol (2mL) was slowly added dropwise, and the reflux reaction was continued. The reaction process was analyzed by TLC monitor. After the reaction was completed, it was quenched by adding saturated aqueous ammonium chloride solution (10 mL), and then extracted with dichloromethane (3×10 mL). The dichloromethane solution obtained by the mixed extraction was concentrated under reduced pressure, and then separated by silica gel column chromatography to obtain the target product IIIc as a yellow oil with a yield of 75%.
[0039] 1 H NMR (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com