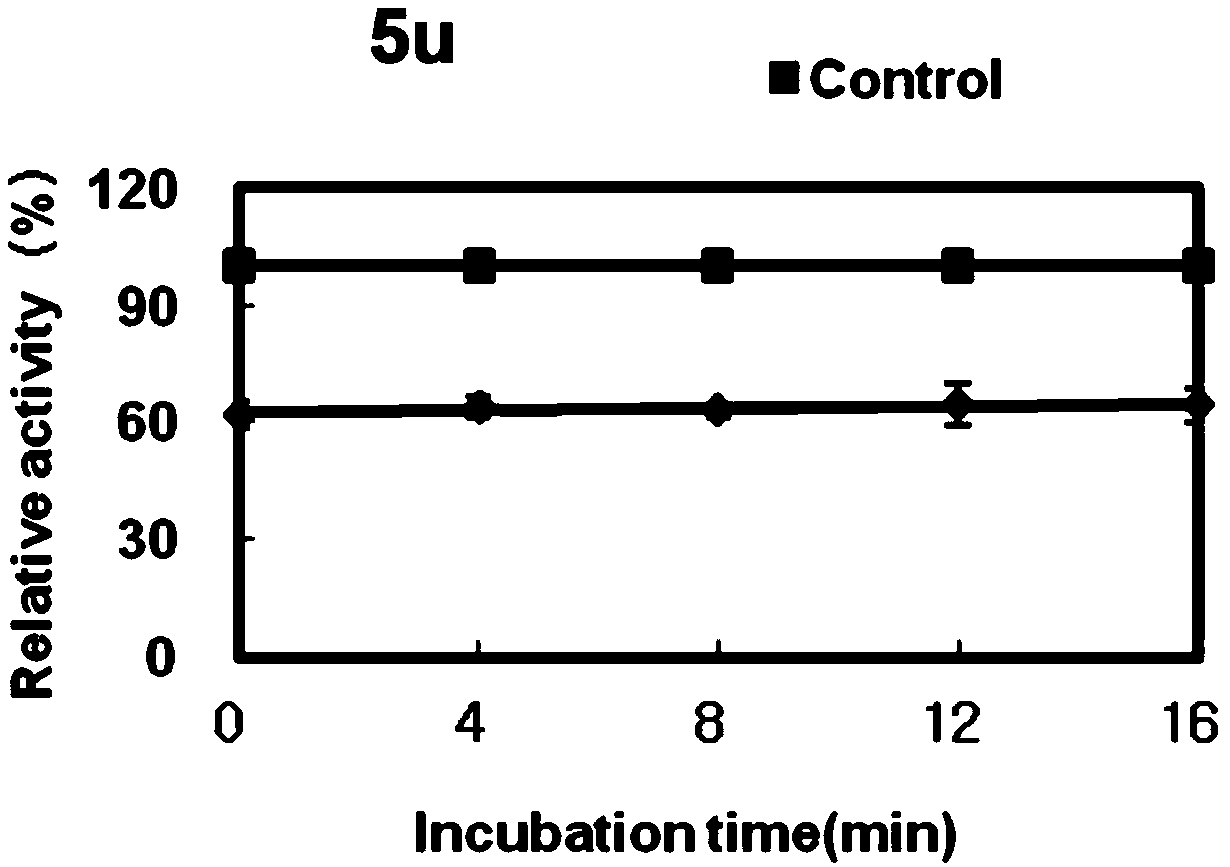
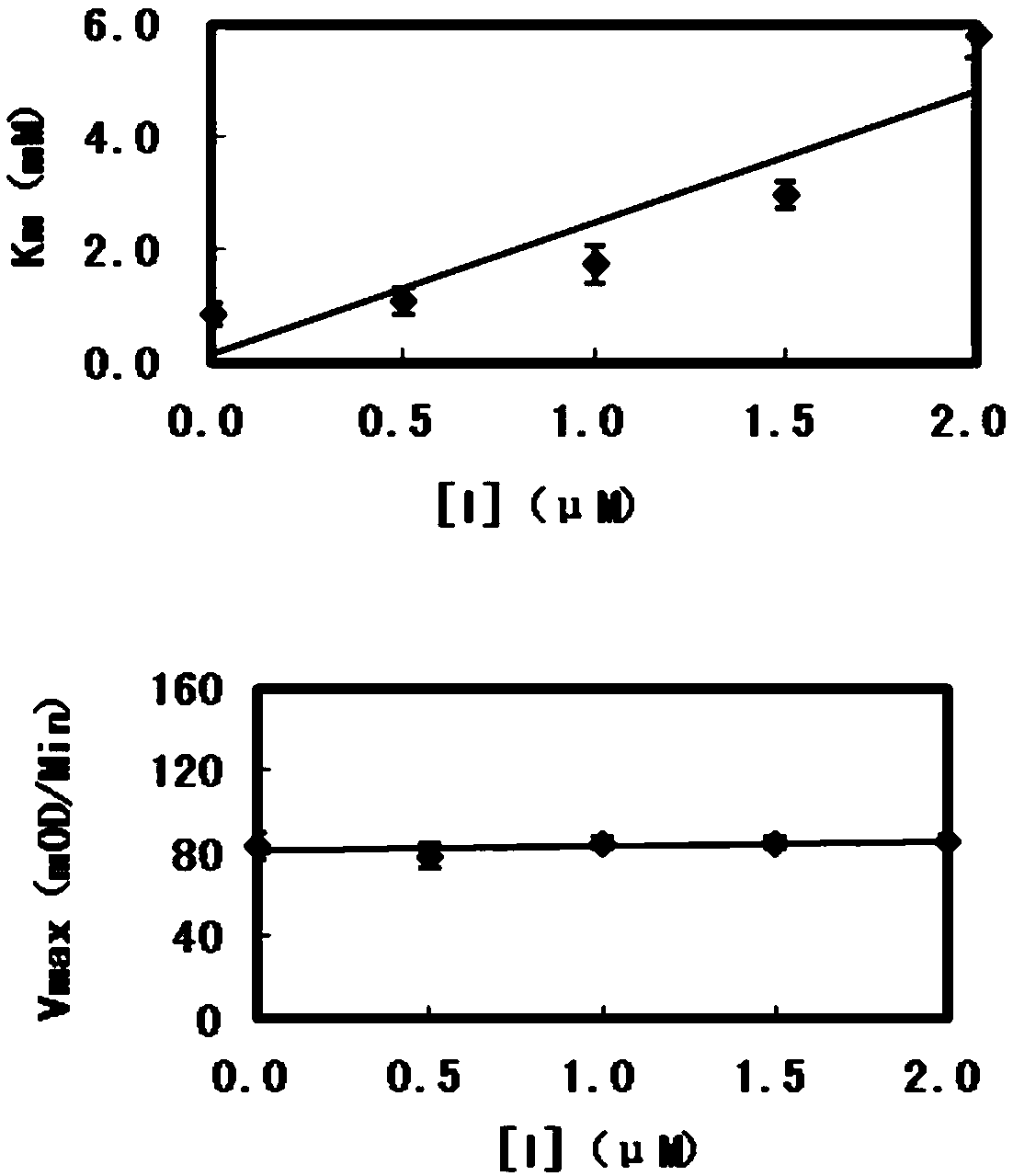
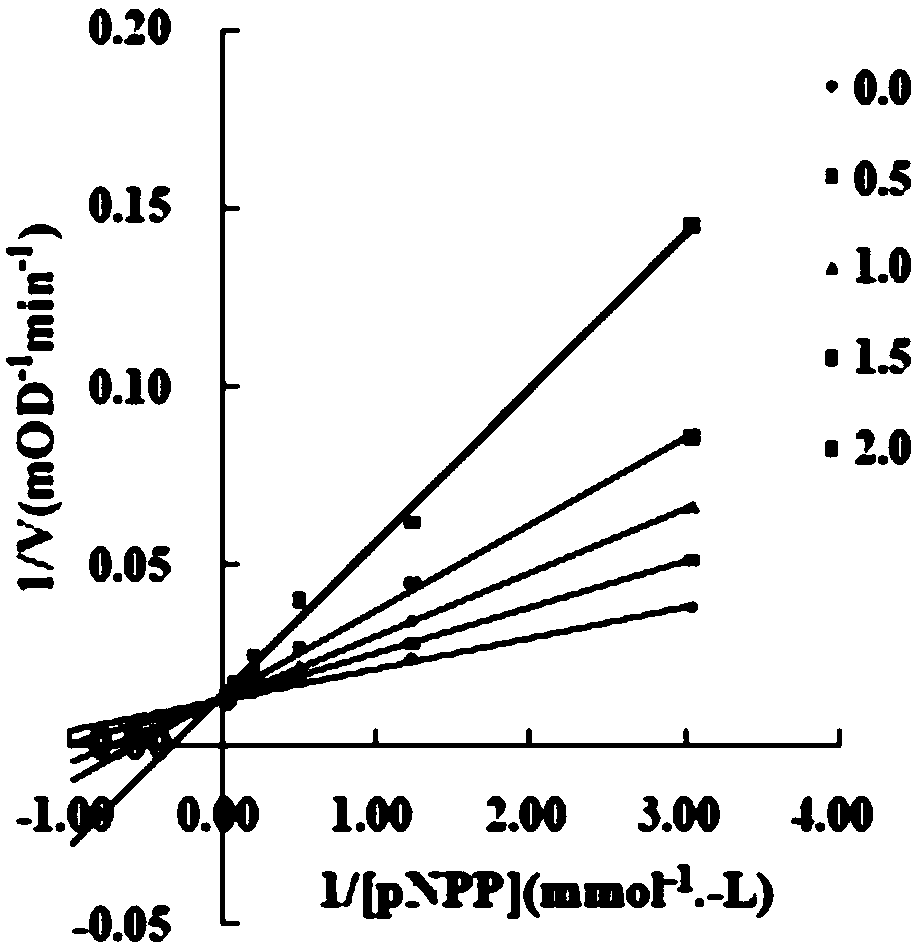
Application of chalcone compound containing carboxymethyl rhodanine structure
A technology of chalcones and compounds, applied in the field of chalcones, can solve the problems of unfavorable effective effects, poor PTPase selectivity, poor bioavailability, etc., achieve high affinity and inhibitory activity, and improve bioavailability , the effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: 2-(Z)-5-(4-((E)-3-naphthyl-3-oxo-1-propenyl)benzylidene)-2-thio-4-thiazolidine Keto-3-yl)acetic acid (5a)
[0029] Glycine (10mmol, 0.75g) was dissolved in 15mL water, NaOH (0.8g, 20mmol) was added, and 0.6mL CS was slowly added dropwise 2 (10 mmol), stirred at room temperature for 10 hours. Anhydrous sodium chloroacetate (0.82 g, 10 mmol) was added in batches to the reaction solution, and stirring was continued at room temperature for 3 hours. Add 6M HCl (30mL), N 2 Protection, stirring and reflux for 16 hours, cooling. The reaction solution was extracted with ethyl acetate (3*50mL), the organic phases were combined, dried over anhydrous magnesium sulfate, filtered, and the filtrate was spin-dried to obtain a yellow-green oil. The crude product was subjected to silica gel column chromatography (eluent: dichloromethane: Methanol = 20:1) to obtain 1.24 g of white solid (2a), with a yield of 65%.
[0030] Dissolve terephthalaldehyde (1.34g, 10.0mmol) in 10...
Embodiment 2
[0032]Example 2: 2-(Z)-5-(4-((E)-3-(3-bromophenyl)-3-oxo-1-propenyl)benzylidene)-2-thio -4-thiazolidinon-3-yl)acetic acid (5b)
[0033] 2'-Naphthophenone (1.70g, 10mmol) was replaced by 3-bromoacetophenone (1.99g, 10mmol), the synthesis method was the same as 5a, and the yellow solid (5b) was 0.20g, the yield was 41%; m.p.289- 290℃.IR(KBr)cm -1 :3449(OH),1687(C=O). 1 HNMR (DMSO-d 6 ,300MHz,ppm)δ:4.53(s,2H,CH 2 ), 7.53(d, J=15.3Hz, 1H, CH=CH), 7.87(s, 1H, CH), 8.03(d, J=15.3Hz, 1H, CH=CH), 7.55-8.36(m, 8H ,Ar—H),13.56(s,1H,COOH). 13 C NMR (DMSO-d 6 ,300MHz,ppm)δ:193.33,188.20,167.19,167.06,143.72,139.83,137.16,136.39,135.32,132.44,131.50,130.40,128.04,123.92,122.83,47.14.9 m / z+
Embodiment 3
[0034] Example 3: 2-(Z)-5-(4-((E)-3-(3-chlorophenyl)-3-oxo-1-propenyl)benzylidene)-2-thio -4-thiazolidinon-3-yl)acetic acid (5c)
[0035] 2'-Naphthophenone (1.70g, 10mmol) was replaced by 3-chloroacetophenone (1.54g, 10mmol), and the synthesis method was the same as that of 5a to obtain 0.16g of yellow solid (5c), with a yield of 36%; m.p.286– 287℃.IR(KBr)cm –1 :3449(OH),1687(C=O). 1 HNMR (DMSO–d 6 ,300M Hz,ppm):δ4.52(s,2H,CH 2 ),7.60(d,J=15.3Hz,1H,CH=CH),7.88(s,1H,CH),7.78(d,J=15.3Hz,1H,CH=CH),7.62–8.24(m,8H ,Ar–H),13.55(s,1H,COOH).MSm / z 444(M+1).Anal.Calcd.for C 21 h 14 ClNO 4 S 2 : C, 56.82; H, 3.18; N, 3.16; S, 14.45. Found: C, 56.80; H, 3.29; N, 3.06; S, 14.63.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com