Tumor targeting drug and preparation method thereof
A tumor-targeting and anti-tumor drug technology, applied in the field of tumor-targeting drugs and their preparation, can solve the problems of complex preparation process, poor biocompatibility of polymer nanocarriers, and influence on normal cells, so as to avoid immune response, improve The effect of tumor treatment, the effect of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of Target Tumor Drugs
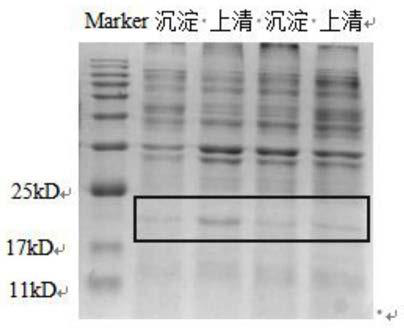
[0032] 1. Synthesize the gene sequence targeting the tumor vector by gene synthesis: obtain the gene sequence encoding the hepatitis B virus-like particle (144 amino acid gene sequence at the C-terminal), and make the gene sequence encoding the RGD polypeptide with tumor-targeting function replace the encoding hepatitis B The gene sequence of amino acids 78-82 at the C-terminal of the virus-like particle, and the gene sequence encoding the connecting peptide is inserted between the gene sequence encoding the hepatitis B virus-like particle and the gene sequence encoding the RGD polypeptide, at the end of the C-terminus of the encoding hepatitis B virus-like particle The gene sequence is connected to the gene sequence encoding the pH-sensitive polyhistidine polypeptide to obtain the recombinant gene sequence targeting the tumor vector; the amino acid sequence of the tumor targeting vector corresponding to one of the recombina...
Embodiment 2
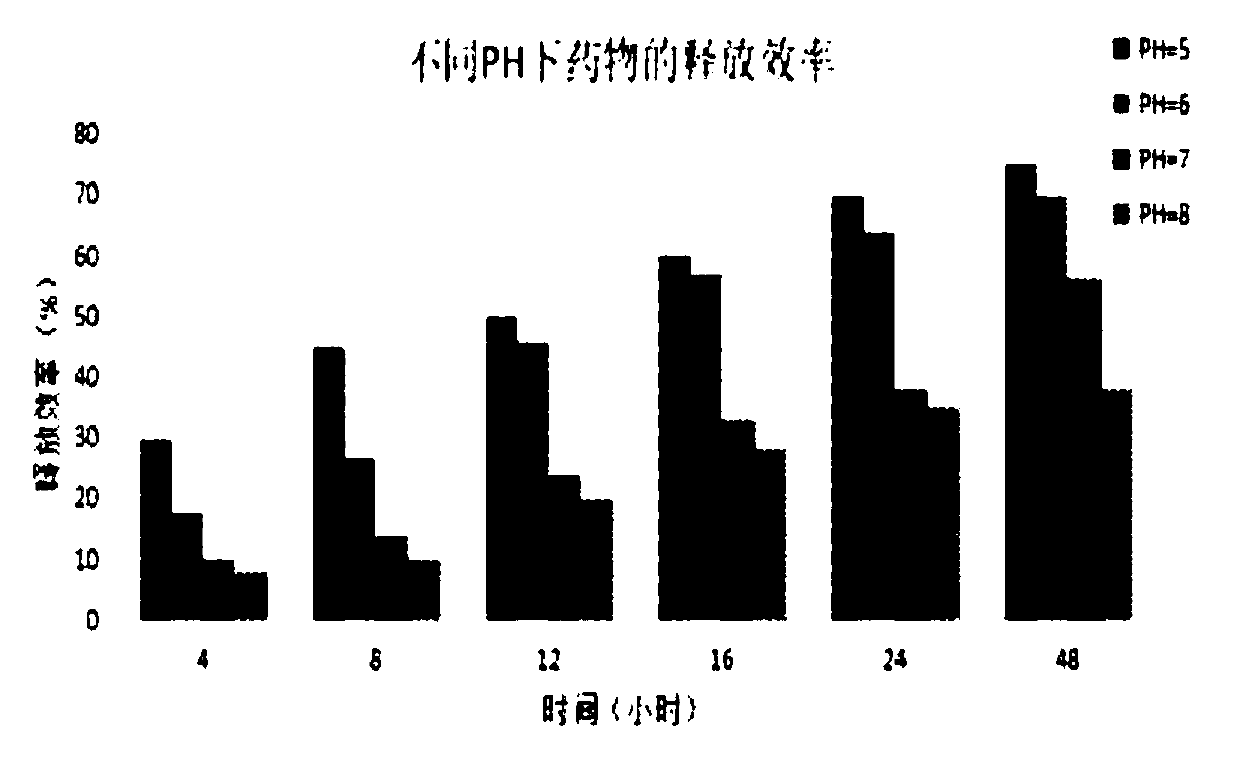
[0073] Example 2: The remaining steps are the same as in Example 1. When loading the drug, take 1 mL of the target protein (~1 mg / mL) recombined in step (2) and incubate with the pre-prepared dissociation solution at 4°C for 2.5 h, the total volume of the solution is 10 mL. At this time, 5 mg of doxorubicin was added to the above dissociation solution, the pH was adjusted to the pKa of doxorubicin, and the resulting mixed solution was continued to co-cultivate for 4 hours with slight shaking. Afterwards, it was transferred to a dialysis bag with a molecular weight of 8,000-14,000 Da, first placed in recombination buffer 1 and dialyzed overnight at 4°C, and replaced with recombination buffer 2 after 12 hours. The dialysis time was 48 hours in total, during which the buffer solution was changed twice to obtain the tumor-targeted drug, which was stored at -20°C.
Embodiment 3
[0074] Example 3: The remaining steps are the same as in Example 1. When loading the drug, take 1 mL of the target protein (~1 mg / mL) reconstituted in step (2) and incubate with the pre-prepared dissociation solution at 4°C for 2.5 h , the total volume of the solution is 10 mL. At this time, 1000 mg of doxorubicin was added to the above dissociation solution, the pH was adjusted to the pKa of doxorubicin, and the resulting mixed solution was continued to co-cultivate for 2 h with slight shaking. Afterwards, it was transferred to a dialysis bag with a molecular weight of 8,000-14,000 Da, first placed in recombination buffer 1 and dialyzed overnight at 4°C, and replaced with recombination buffer 2 after 12 hours. The dialysis time was 48 hours in total, during which the buffer solution was changed twice to obtain the tumor-targeted drug, which was stored at -20°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com