Application method of lanthanum titanate nano-sheet to photocatalysis nitrogen fixation
An application method and nanosheet technology, applied in the direction of chemical instruments and methods, applications, titanate, etc., can solve problems such as low efficiency and achieve stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
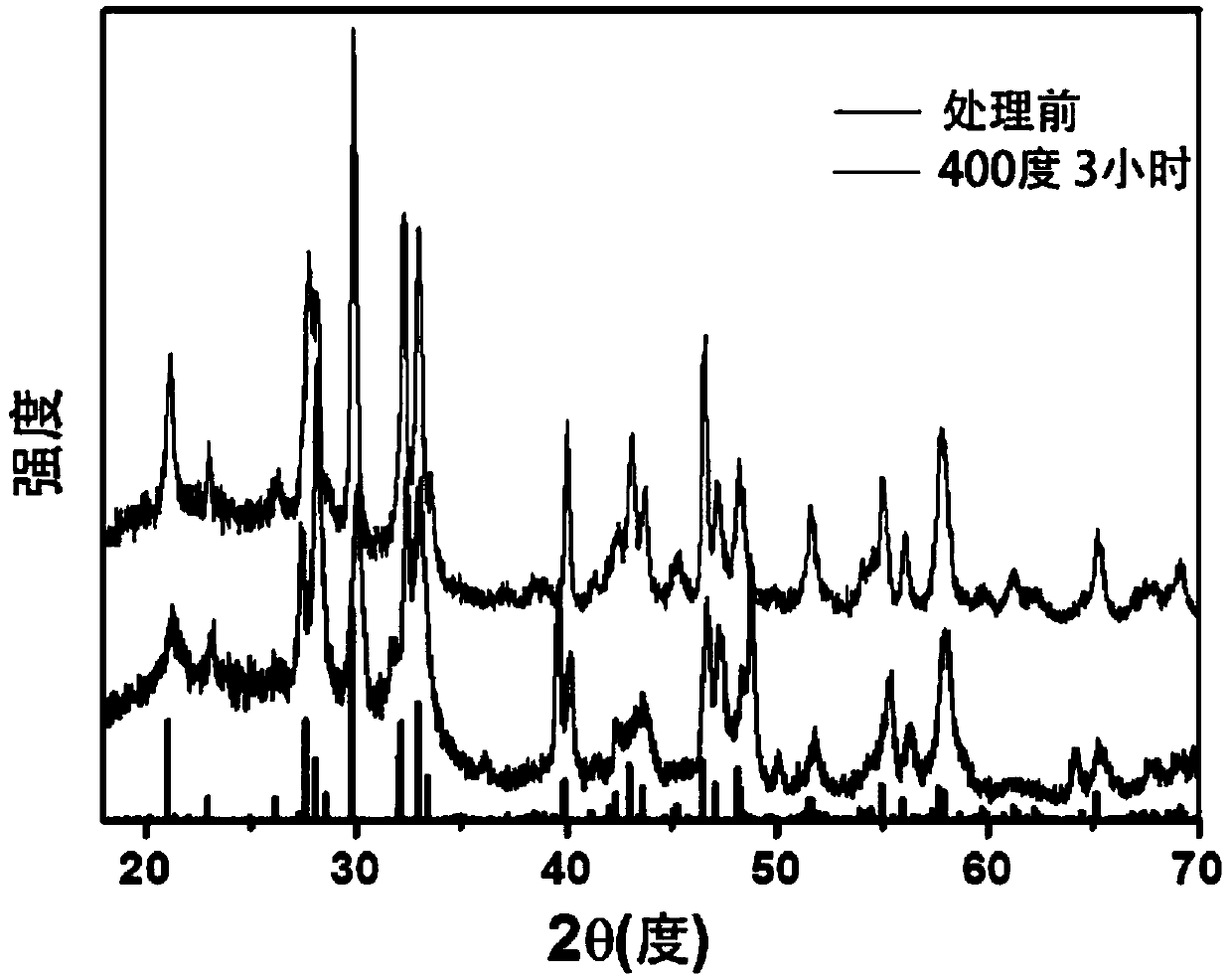
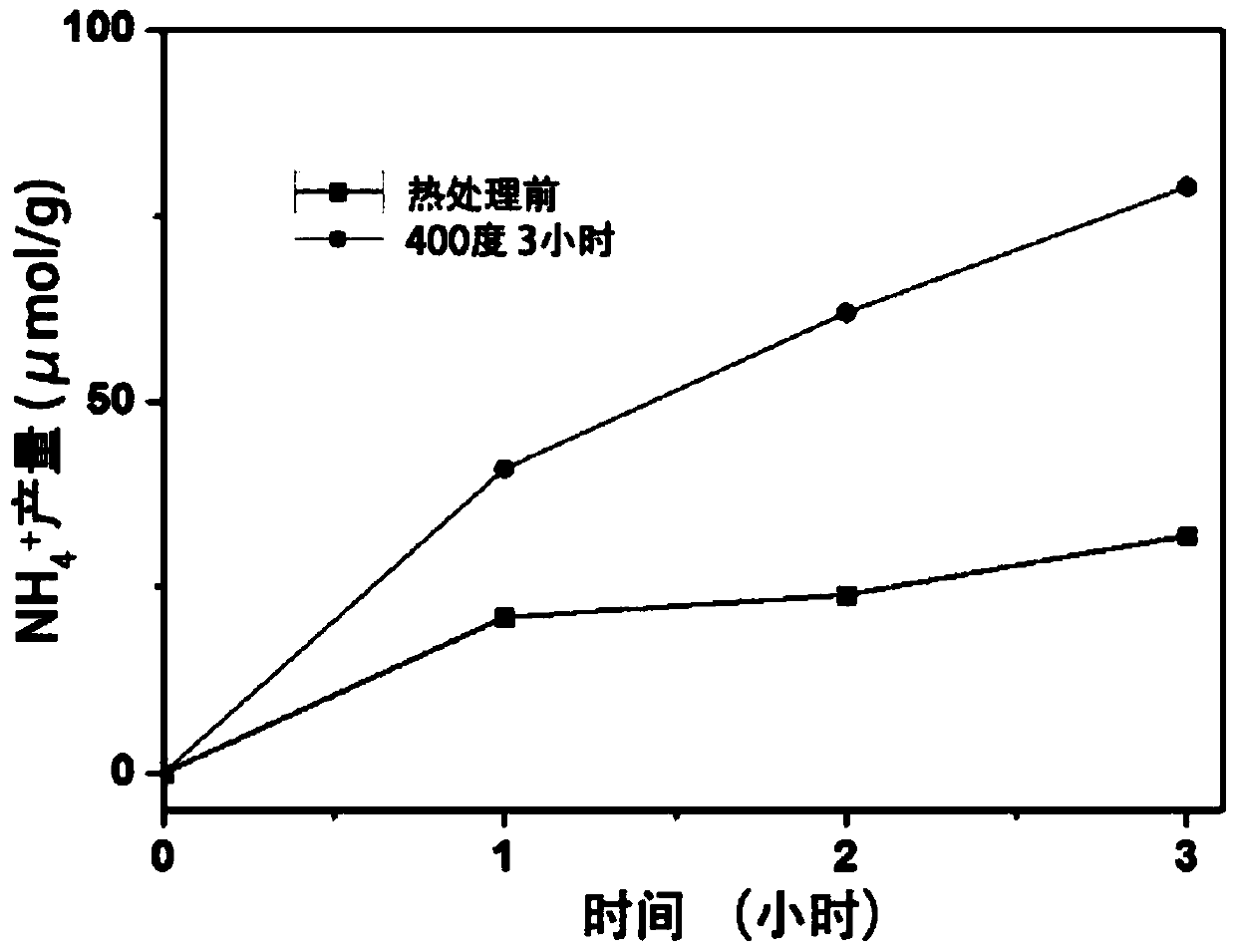
[0053] First weigh 200mg of the prepared La 2 Ti 2 o 7 That is, LTO was heat-treated at 400°C for 3 hours under an argon-hydrogen atmosphere (5% hydrogen + 95% argon) to prepare LTO samples containing oxygen vacancies, and the gas flow rate was 0.2 L / min.
[0054] Then weigh 30 mg of the LTO sample before and after heat treatment, add 30 mL of deionized water to the reaction test tube, connect it to the gas cylinder, plug the rubber stopper, light, nitrogen, the flow rate is 0.1 L / min, and draw 3 mL every 1 h The reaction solution was centrifuged to obtain a supernatant for later use. Take 1mL supernatant and mix with prepared 1mL Nessler's Reagent (Nessler's Reagent), put it into a spectrophotometer to measure the absorbance, take NH 4+ The absorbance value record at the 420nm place of the absorbance maximum position, and then compare with the absorbance of the ammonia nitrogen standard solution, finally obtain the concentration of ammonia nitrogen in the reaction solution...
Embodiment 2
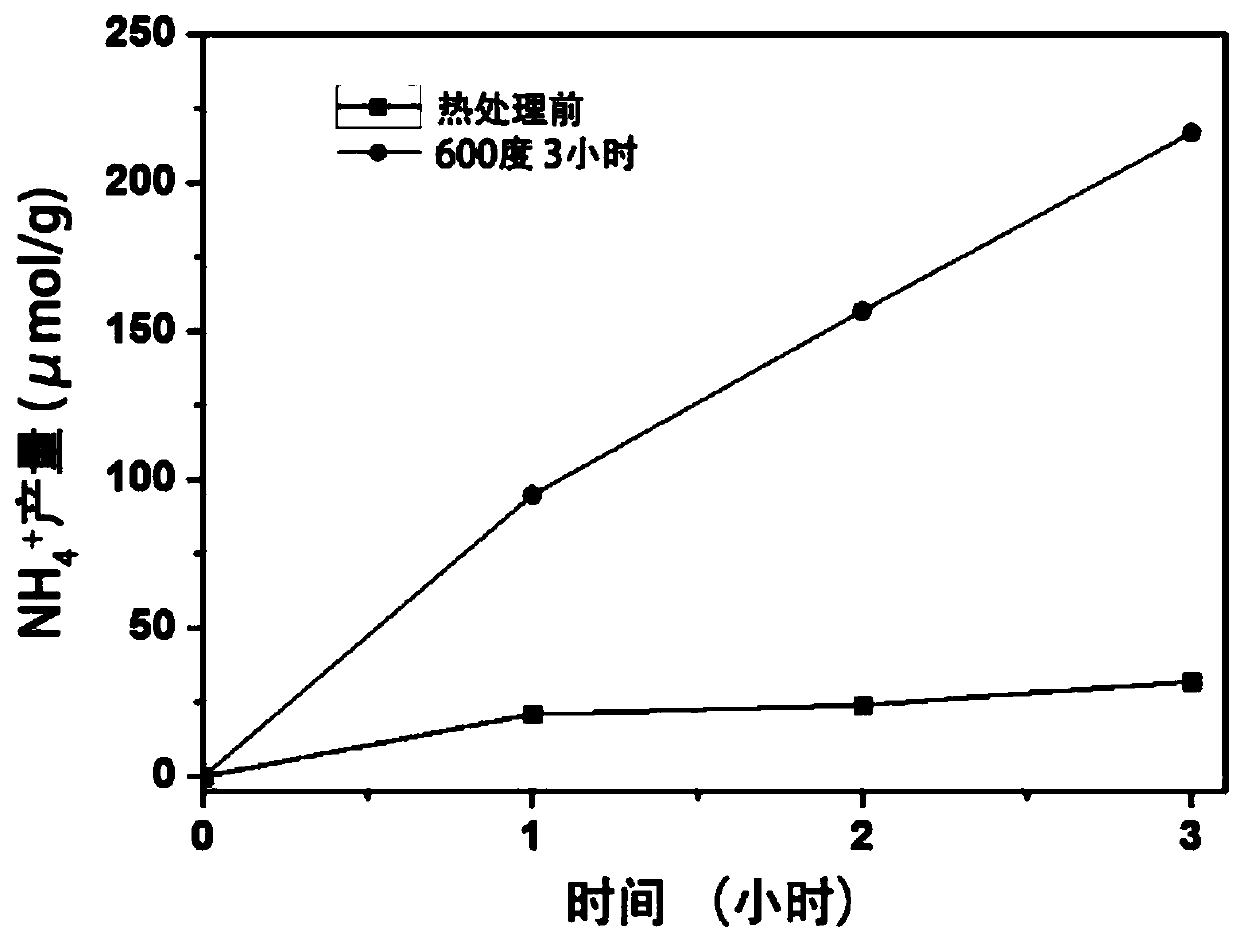
[0056] First, weigh 200 mg of the prepared LTO and heat-treat it at 600 degrees for 3 hours under an argon-hydrogen atmosphere (5% hydrogen + 95% argon) to prepare an LTO sample containing oxygen vacancies, with a gas flow rate of 0.2 L / min;
[0057] Then weigh 30 mg of the LTO sample before and after heat treatment, add 30 mL of deionized water to the reaction test tube, connect it to the gas cylinder, plug the rubber stopper, light, nitrogen, the flow rate is 0.1 L / min, and draw 3 mL every 1 h The reaction solution was centrifuged to obtain a supernatant for later use. Take 1mL supernatant and mix it with 1mL Nessler's Reagent, put it into a spectrophotometer to measure the absorbance, take NH 4+ The absorbance value record at the 420nm place of the absorbance maximum position, and then compare with the absorbance of the ammonia nitrogen standard solution, finally obtain the concentration of ammonia nitrogen in the reaction solution, by image 3 It can be seen that the nitr...
Embodiment 3
[0059] First, weigh 200 mg of the prepared LTO and heat-treat it at 600 degrees for 6 hours under an argon-hydrogen atmosphere (5% hydrogen + 95% argon) to prepare an LTO sample containing oxygen vacancies, with a gas flow rate of 0.2 L / min;
[0060] Then weigh 30 mg of the LTO sample before and after heat treatment, add 30 mL of deionized water to the reaction test tube, connect it to the gas cylinder, plug the rubber stopper, light, nitrogen, the flow rate is 0.1 L / min, and draw 3 mL every 1 h The reaction solution was centrifuged to obtain the supernatant for later use; take 1mL of the supernatant and mix it with 1mL of the prepared Nessler's Reagent (Nessler's Reagent), put it into a spectrophotometer to measure the absorbance, and take NH 4+ The absorbance value record at the 420nm place of the absorbance maximum position, and then compare with the absorbance of the ammonia nitrogen standard solution, finally obtain the concentration of ammonia nitrogen in the reaction sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com