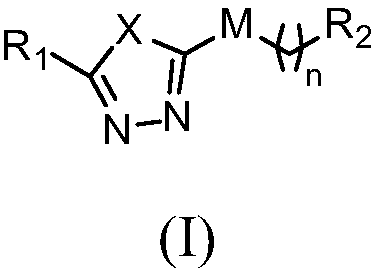
1,3,4-oxadiazole (thiadiazole) imidazole compound as well as preparation method and use thereof
A compound, imidazole technology, applied in the field of imidazole compounds and their preparation, can solve the problem of insufficient activity and efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
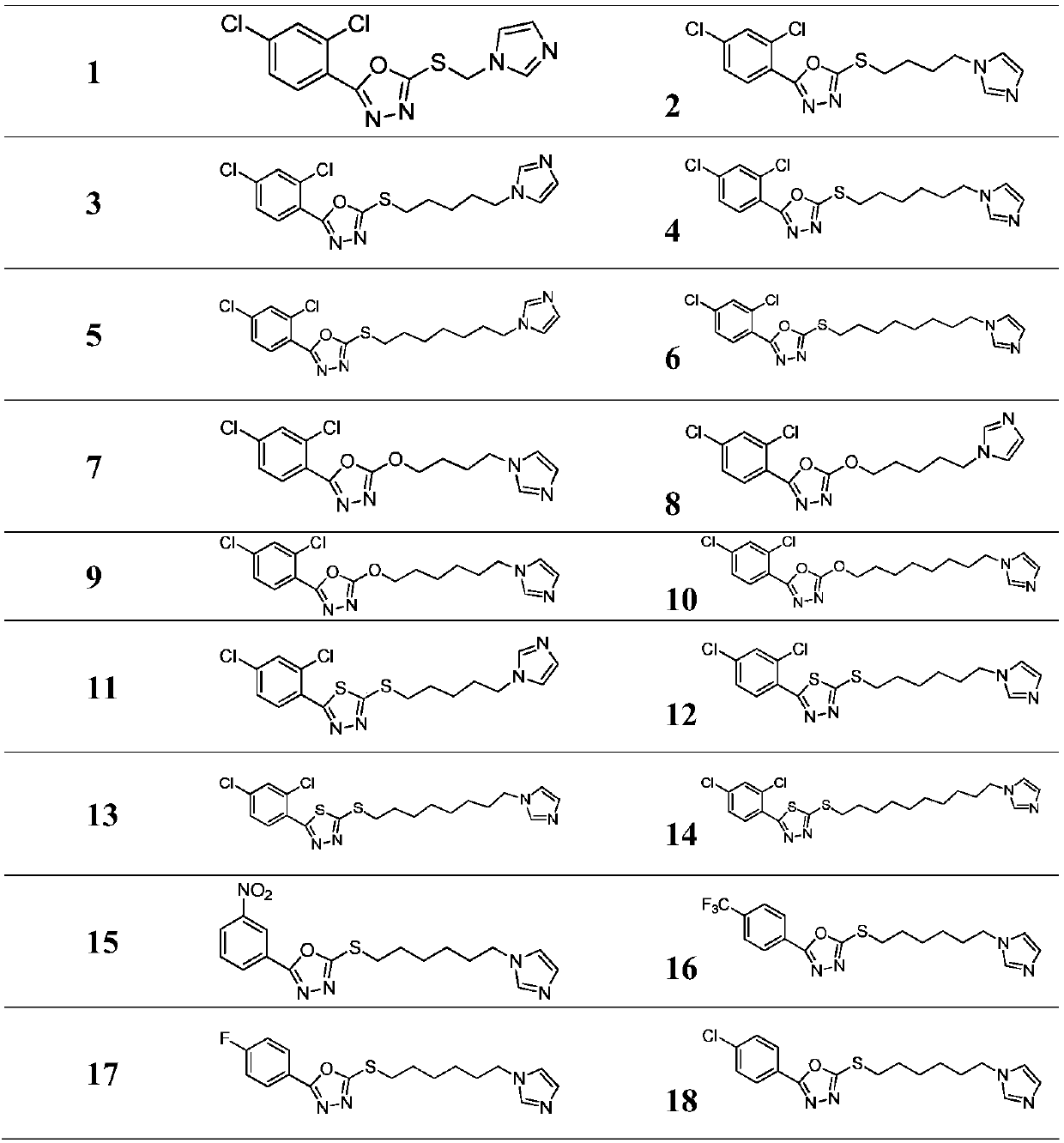
[0082]Example 1: Preparation of intermediate 2-((4-bromobutyl)thio)-5-(2,4-dichlorophenyl)-1,3,4-oxadiazole
[0083] Dissolve 1g (4.05mmol) of 5-(2,4-dichlorophenyl)-1,3,4-oxadiazole-2-thiol and 1.4g (10.13mmol) of potassium carbonate in 20mL of DMF, and stir for 20min. Weigh 1.75g (8.1mmol) of 1,4-dibromobutane in the above-mentioned reaction flask, react at room temperature for 2 hours, stop the reaction, extract with ethyl acetate, wash with saturated ammonium chloride, dry, remove the solvent, and perform column chromatography to obtain 1.3 g pale yellow liquid, yield 86.7%.
[0084] With the steps similar to Example 1, intermediates such as halogenated thiadiazole sulfides, halogenated oxadiazole oxyethers, and halogenated oxadiazole sulfides were prepared using corresponding raw materials.
Embodiment 2
[0085] Example 2: Target compound 2-((4-(1H-imidazol-1-yl)butyl)thio)-5-(2,4-dichlorophenyl)-1,3,4-oxadiazole preparation of
[0086] Dissolve 29mg (1.21mmol) of sodium hydride and 65mg (0.88mmol) of imidazole in 4mL of anhydrous DMF, stir for 15min, weigh 0.3g (0.79mmol) of 2-((4-bromobutyl)thio)-5- (2,4-dichlorophenyl)-1,3,4-oxadiazole was placed in the above reaction flask, reacted at room temperature for 8 hours, and then stopped the reaction, extracted with ethyl acetate, washed with saturated ammonium chloride, dried, precipitated, and column layer Analysis gave 0.21 g of light yellow solid with a yield of 71.7% and a melting point of 61-63°C.
[0087] The target compounds such as thiadiazole sulfide, oxadiazole oxyether, pyrazole, pyridine, piperidine, morpholine, triazole, diethylamine, etc. were prepared by the similar steps of Example 2 using corresponding raw materials.
Embodiment 3
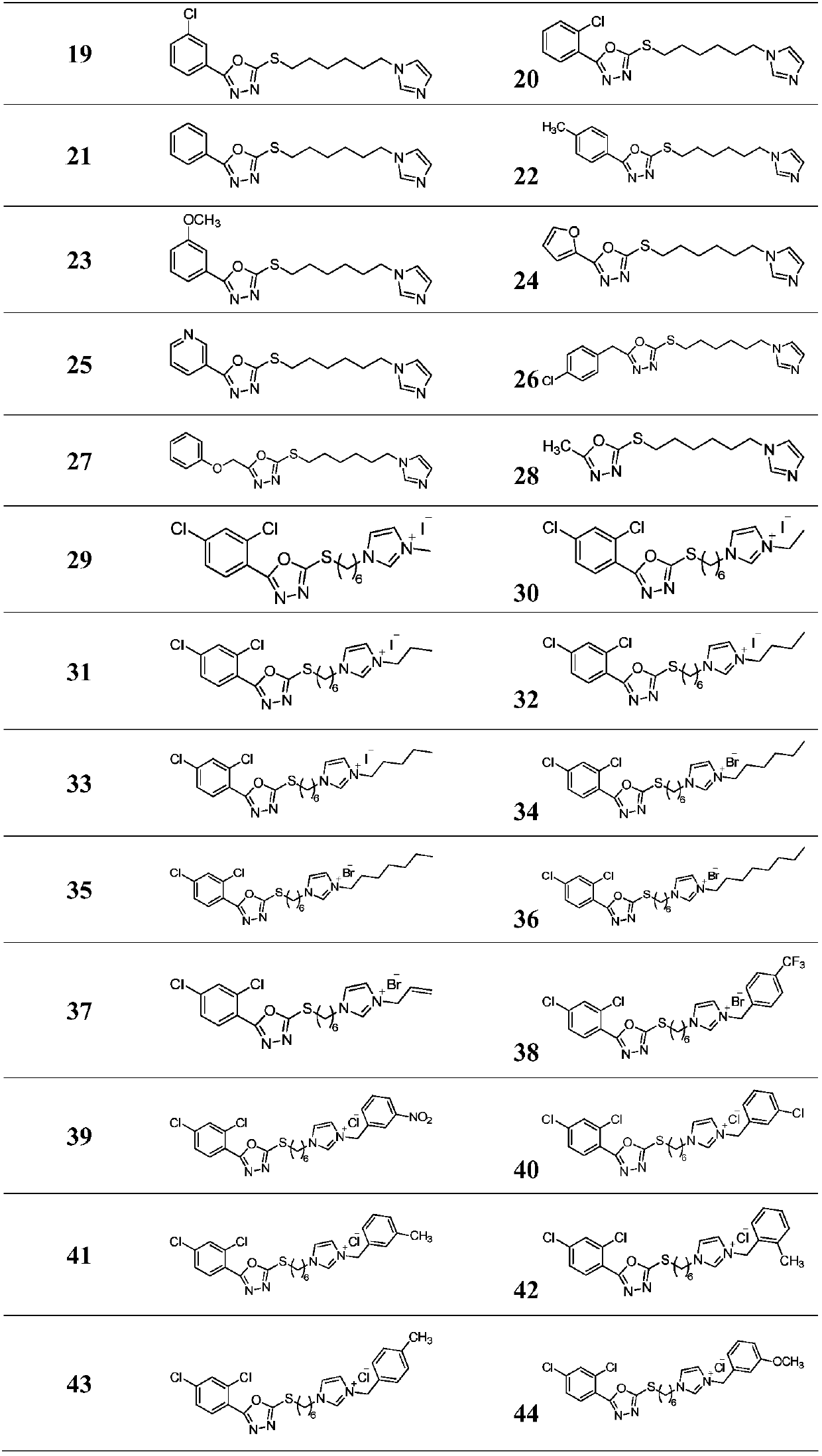
[0088] Example 3: Target compound 1-(6-((5-(2,4-dichlorophenyl)-1,3,4-oxadiazol-2-yl)thio)hexyl)-3-methyl - Preparation of 1H-imidazole-3-iodide
[0089] 0.17g (0.43mmol) 2-((6-(1H-imidazol-1-yl)hexyl)thio)-5-(2,4-dichlorophenyl)-1,3,4-oxadiazole and 0.18g (1.29mmol) of methyl iodide were dissolved in 4mL of acetonitrile, reacted at 70°C for 12h, then stopped the reaction, precipitation, and column chromatography gave 0.19g of yellow liquid with a yield of 83.7%.
[0090] The other target compounds were prepared by using the corresponding raw materials in the similar steps of Example 3.
[0091] The structure, H NMR spectrum and carbon spectrum data of the 1,3,4-oxa(thia)diazolyl pyridinium salt compound are shown in Table 1, and the physicochemical properties are shown in Table 2.
[0092] Table 1 H NMR spectrum, carbon spectrum and fluorine spectrum data of some compounds
[0093]
[0094]
[0095]
[0096]
[0097]
[0098]
[0099]
[0100]
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com