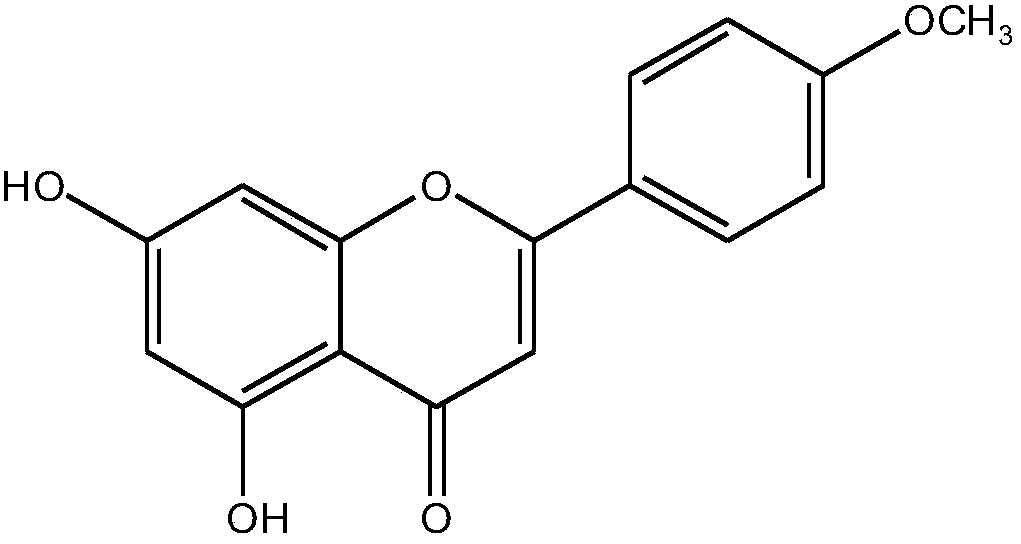
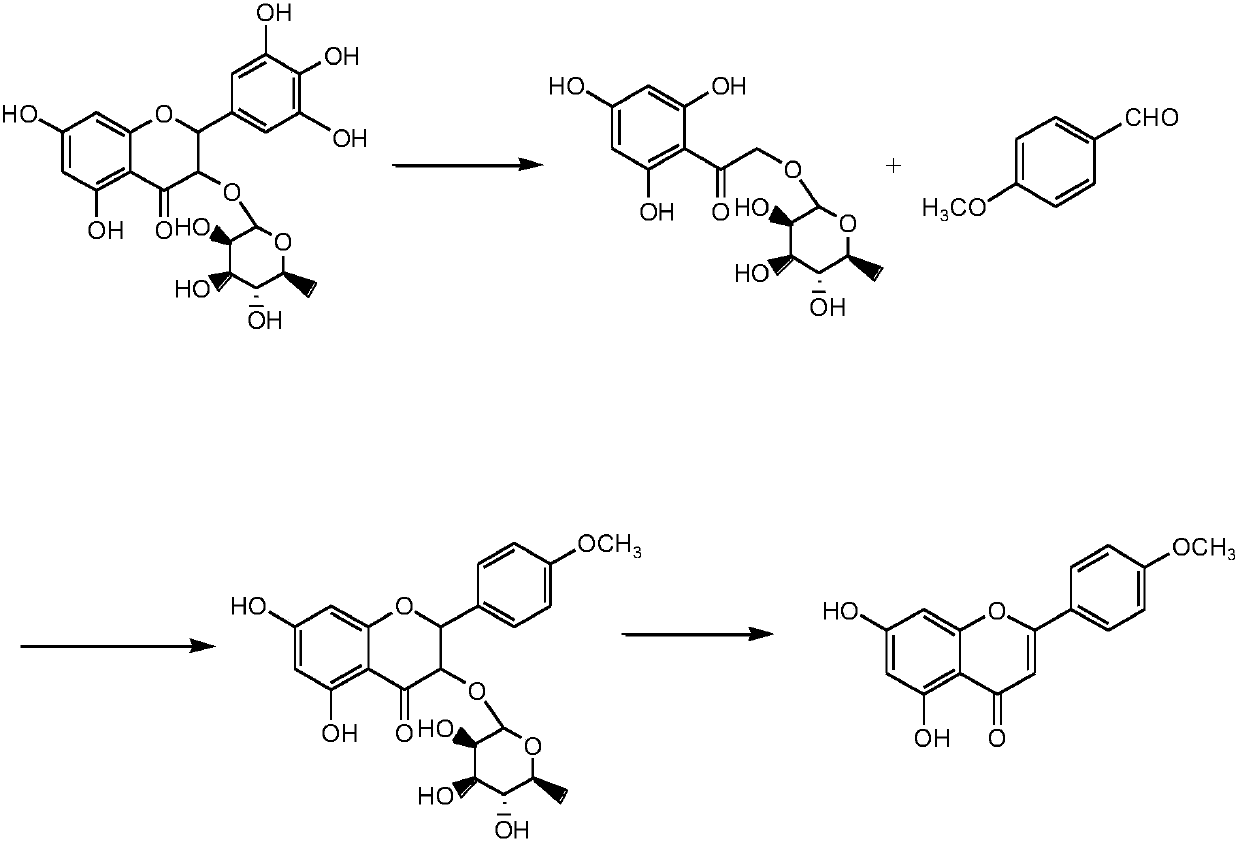
Preparation method of acacetin
A technology of acacetin and intermediates, which is applied in the field of preparation of raw materials, can solve the problems of being unsuitable for large-scale industrial production, harsh reaction conditions, and unfriendly environment, and achieve the effects of abundant supply, high reaction efficiency, and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Step 1) hydrolysis prepares hydroxyacetophenone intermediate
[0042] 1.1) Put 50g of dihydromyricetin with a liquid phase content of more than 98% into the reactor, add 500g of sodium hydroxide solution with a mass fraction of 15%, stir to dissolve, mix evenly, heat up and reflux, and carry out hydrolysis reaction; high-efficiency liquid phase Chromatographic monitoring, with no increase in the hydroxyacetophenone intermediate as the reaction control end point, after 1.5 hours, stop the reaction. Cool down to room temperature, and slowly dropwise add hydrochloric acid solution with a mass concentration of 50% to the reaction solution, adjust the pH to 6.2, stir for 2 hours, let stand for 1 hour, and filter to obtain a light yellow solid;
[0043] 1.2) Add 400g of water to the light yellow solid to reflux for 1 hour, stand at room temperature and filter, then add 400g of water to reflux for 1 hour, stand at room temperature, filter, and then dry to obtain the hydroxyace...
Embodiment 2
[0053] Step 1) hydrolysis prepares hydroxyacetophenone intermediate
[0054] 1.1) Put 50g of dihydromyricetin with a liquid phase content of more than 98% into the reactor, add 420g of sodium hydroxide solution with a mass fraction of 17.5%, stir to dissolve, mix evenly, heat up and reflux, and carry out hydrolysis reaction; high-efficiency liquid phase Chromatographic monitoring, with no increase in the hydroxyacetophenone intermediate as the reaction control end point, after 1.5 hours, stop the reaction. Cool down to room temperature, and slowly dropwise add hydrochloric acid solution with a mass concentration of 50% to the reaction solution, adjust the pH to 6.4, stir for 2 hours, let stand for 1 hour, and filter to obtain a light yellow solid;
[0055] 1.2) Add 480g of water to the light yellow solid to reflux for 1 hour, stand at room temperature and filter, then add 480g of water to reflux for 1 hour, stand at room temperature, filter, and then dry to obtain the hydroxya...
Embodiment 3
[0065] Step 1) hydrolysis prepares hydroxyacetophenone intermediate
[0066] 1.1) Put 50g of dihydromyricetin with a liquid phase content of more than 98% into the reactor, add 480g of sodium hydroxide solution with a mass fraction of 17.5%, stir to dissolve, mix evenly, heat up and reflux, and carry out hydrolysis reaction; high-efficiency liquid phase Chromatographic monitoring, with no increase in the hydroxyacetophenone intermediate as the reaction control end point, after 1.5 hours, stop the reaction. Cool down to room temperature, and slowly dropwise add hydrochloric acid solution with a mass concentration of 50% to the reaction solution, adjust the pH to 6.4, stir for 2 hours, let stand for 1 hour, and filter to obtain a light yellow solid;
[0067] 1.2) Add 560g of water to the light yellow solid to reflux for 1 hour, stand at room temperature and filter, then add 560g of water to reflux for 1 hour, stand at room temperature, filter, and then dry to obtain the hydroxya...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com