Method for producing chloroformate compound
A manufacturing method and technology of chloroformate, applied in the preparation of organic carbonate, preparation of phosgene or haloformate, organic chemistry, etc., can solve problems such as phosgene gas release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0056] Hereinafter, the present invention will be described more specifically with examples given, but the present invention is of course not limited to the following examples, and can be implemented with appropriate changes within the scope of the aforementioned / later-described gist, All of them are included in the technical scope of the present invention.
reference example 1
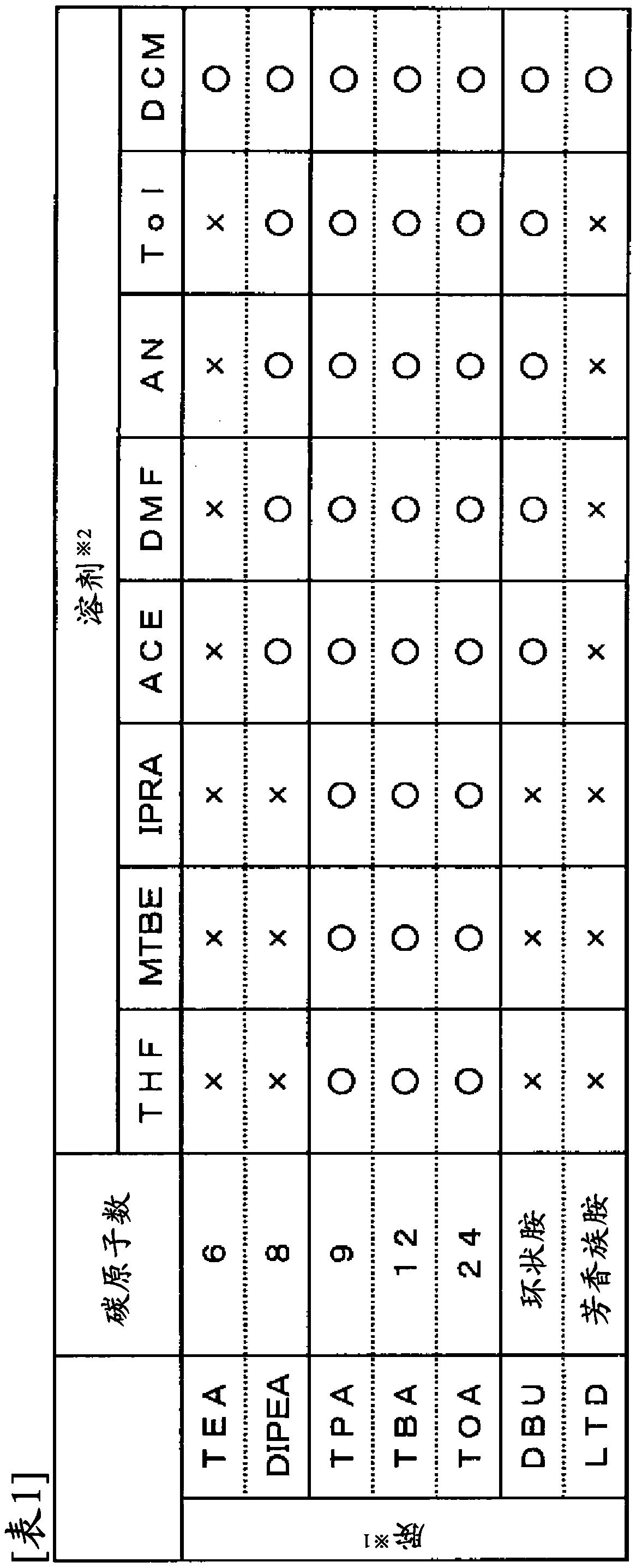
[0058] 1 g of various amines shown in Table 1 below was added to 10 mL of each solvent shown in Table 1 below to prepare a solution with a concentration of 10% (weight / volume). A n-propanol solution of hydrochloric acid (hydrochloric acid concentration: 34% by weight) was added to this solution until it became 1.1 equivalent (=molar amount of hydrogen chloride / molar amount of amine), and stirred at room temperature for 1 hour. After stirring, the presence or absence of solid precipitation was confirmed visually. In the table, ○ means that no solid was precipitated, and x means that a solid was precipitated.
[0059]
[0060] *1: Using amine
[0061] Triethylamine (TEA), diisopropylethylamine (DIPEA), tripropylamine (TPA), tributylamine (TBA), trioctylamine (TOA), 1,8-diazabicyclo[5,4 ,0] Undec-7-ene (DBU), 2,6-Lutidine (LTD)
[0062] *2: Solvents are used
[0063] Tetrahydrofuran (THF), methyl tert-butyl ether (MTBE), isopropyl acetate (IPRA), acetone (ACE), N,N-dimethy...
Embodiment 1
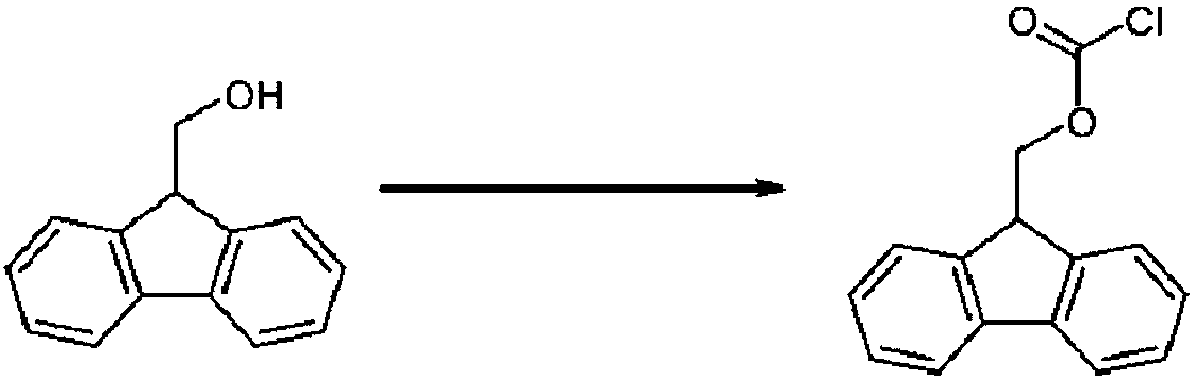
[0075] 25.49 g of toluene was put into 0.61 g of triphosgene to prepare a homogeneous solution, which was used as liquid A. Separately, 22.81 g of toluene was put into 1.42 g of tributylamine and 1.00 g of 9-fluorenylmethanol to prepare a homogeneous solution, which was used as liquid B. Put the T-shaped mixer (inner diameter: 2mm, material: polytetrafluoroethylene (PTFE)) and retention line (tube inner diameter: 2mm, material: polytetrafluoroethylene (PTFE)) in a constant temperature bath at 0°C, and Liquid A and liquid B were fed with a diaphragm pump (manufactured by KNF) at a rate of 2 ml / min (linear velocity: 127 cm / min), mixed with a T-shaped mixer, and circulated in the retention line for 1 minute to react. The reaction liquid was quenched with stirring in 34.85 g of 13% phosphoric acid water charged to the flask, and after liquid separation, 66.24 g of an organic layer containing 1.19 g of 9-fluorenylmethyl chloroformate was obtained (yield 90%). It should be noted th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com