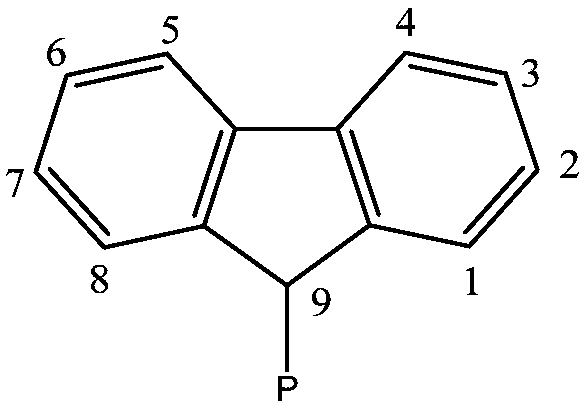
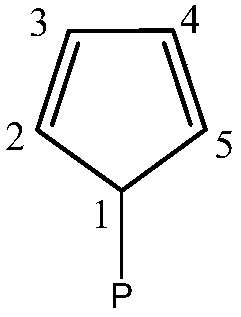
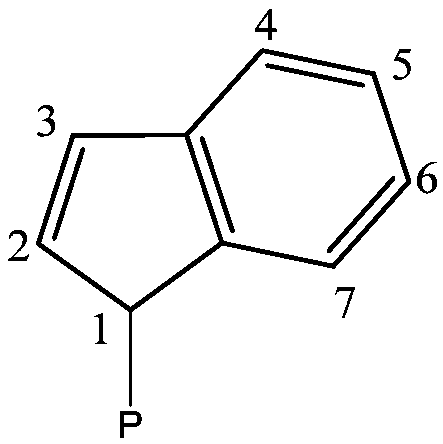
Preformed catalytic system comprising a rare-earth metallocene
一种催化体系、稀土金属的技术,应用在制备所述催化体系领域,能够解决降低生产装置生产量、运行复杂化等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0085] b) Polymer preparation:
[0086] The polymer samples were not specially treated prior to analysis. It was simply dissolved in tetrahydrofuran + 0.1% by volume distilled water at a concentration of about 1 g / l. The solution was then filtered through a filter with a porosity of 0.45 μm before injection.
[0087] c) SEC analysis:
[0088] The apparatus used was a Waters Alliance chromatograph. The elution solvent was tetrahydrofuran, the flow rate was 0.7 ml / min, the system temperature was 35° C. and the analysis time was 90 minutes. A set of four Waters columns in series having the trade designations Styragel HMW7, Styragel HMW6E and two Styragel HT6E was used.
[0089] The volume of the injected polymer sample solution was 100 µl. The detector was a Waters 2410 differential refractor and the software to use the chromatographic data was a Waters Empower system.
[0090] Calculated average molar masses are relative to a calibration curve generated from commercial...
Embodiment 1 to 11
[0092] The catalytic systems C1 to C11 according to the invention were prepared according to the following procedure.
[0093] At the levels shown in Table I, the cocatalyst butyloctylmagnesium (BOMAG) followed by the metallocene [Me 2 Si(Flu) 2 Nd(μ-BH 4 ) 2Li(THF)] was added to a reactor containing the hydrocarbon solvent methylcyclohexane (MCH) or toluene (Tol). The activation time was 10 minutes and the reaction temperature was 20° C. (step a)). Subsequently, the preformed monomers, which are ethylene (Eth), a mixture of ethylene and 1,3-butadiene (Eth / Bde), or a mixture of ethylene and isoprene (Eth / Iso)) into the reactor, the mole fraction of ethylene in the mixture of ethylene and 1,3 butadiene or ethylene and isoprene is 0.8. The preforming reactions were carried out at the temperatures indicated in Table I for the time periods also indicated in Table I. In Example 4, at the end of step b), the reactor was degassed and flushed with nitrogen to remove unconverted...
Embodiment 12 to 14
[0095] The catalytic system CE1-1 not in accordance with the present invention was prepared according to the method disclosed in the patent application WO 2007054224 and described as follows:
[0096] At the levels shown in Table II, the cocatalyst butyloctylmagnesium (BOMAG) followed by the metallocene [Me 2 Si(Flu) 2 Nd(μ-BH 4 ) 2 Li(THF)] was added to the reactor containing toluene (Tol). The activation time is 10 minutes, and the reaction temperature is 20°C. Its preparation conditions are shown in Table II.
[0097] Catalytic system CE1-2 not in accordance with the invention was prepared in a similar manner to catalytic system CE1-1, except that the solvent was methylcyclohexane.
[0098] Catalyst systems CE1-3 not in accordance with the invention were prepared according to the following procedure:
[0099] At the levels shown in Table II, the cocatalyst butyloctylmagnesium (BOMAG) followed by the metallocene [Me 2 Si(Flu) 2 Nd(μ-BH 4 ) 2 Li(THF)] was added to a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com