Novel intermediate for synthesizing teprenone and application of novel intermediate
A technology of teprenone and addition reaction, applied in the field of synthesizing teprenone and new intermediates for teprenone synthesis, can solve the problems of low total yield, high synthesis cost, long synthesis steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
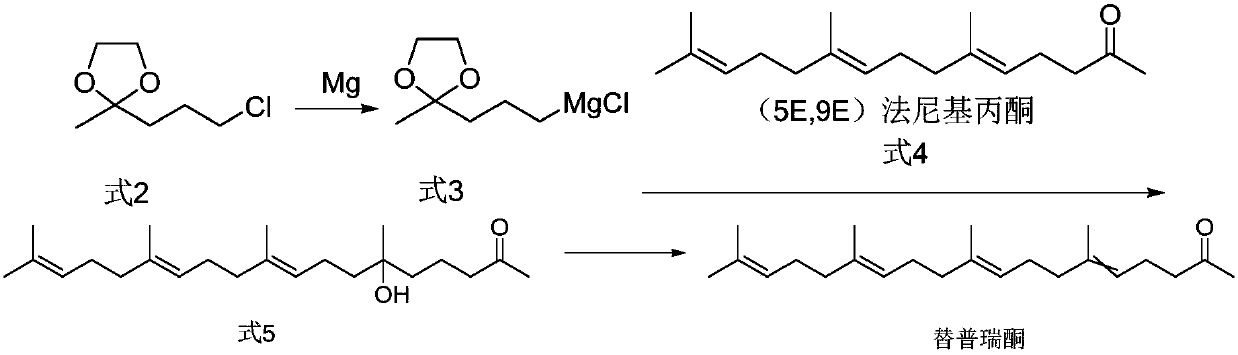
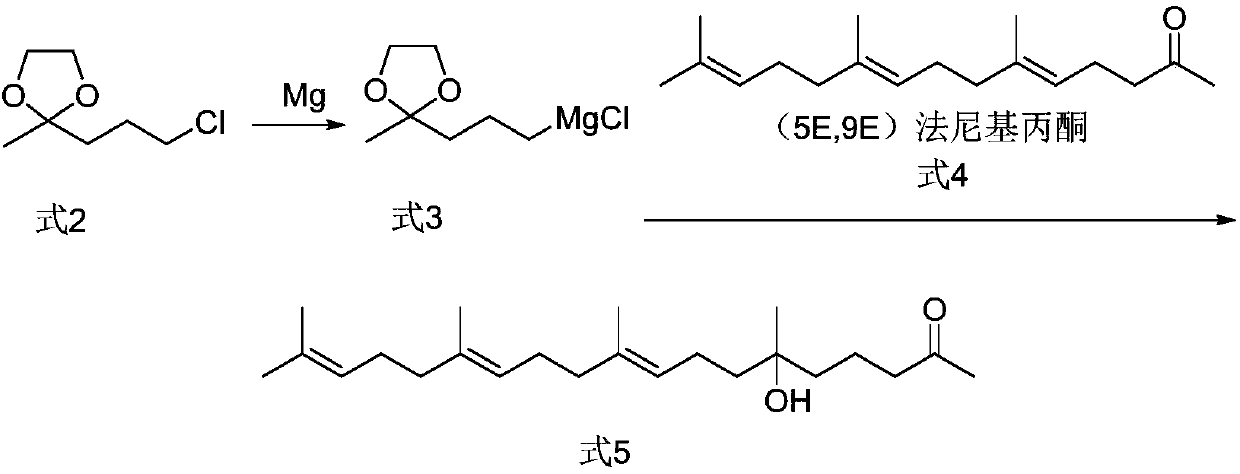
[0056] Embodiment 1. Synthesis of 2-(3-chloropropyl)-2-methyl-1,3-dioxolane (formula 2)
[0057] 16.5 g of 5-chloro-2-pentanone, 150 ml of toluene, 0.5 g of p-toluenesulfonic acid, and 16.9 g of ethylene glycol were added to a 250 ml reaction flask. After the addition, the temperature of the oil bath was raised to reflux and water was separated for 4 hours. The reaction solution was cooled to room temperature and washed with sodium bicarbonate solution and water. The obtained toluene organic layer was distilled at 60° C. under reduced pressure to obtain 19.68 g of oily 2-(3-chloropropyl)-2-methyl-1,3-dioxolane (94.6% yield).
Embodiment 2
[0058] Embodiment 2. Synthesis of 2-(3-chloropropyl)-2-methyl-1,3-dioxolane (formula 2)
[0059] 16.5 g of 5-chloro-2-pentanone, 150 ml of n-hexane, 0.5 g of p-toluenesulfonic acid, and 16.9 g of ethylene glycol were added to a 250 ml reaction flask. After the addition, the oil bath was warmed up and refluxed to separate water for 4 hours. The reaction liquid was cooled to room temperature and washed with sodium bicarbonate solution and water. The obtained n-hexane organic layer was distilled at 60°C under reduced pressure to obtain 18.15 g of oily 2-(3-chloropropyl)-2-methyl-1,3-dioxolane (87.24% yield).
Embodiment 3
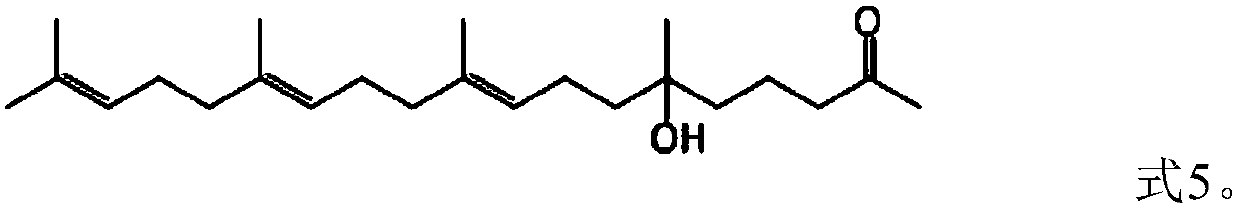
[0060] Example 3. Synthesis of (9E,13E)-6-hydroxyl-6,10,14,18-4methyl nonadeca-9,13,17,-trien-2-one (formula 5)
[0061] Add 1.2 g of magnesium chips, 20 ml of tetrahydrofuran, and one iodine pellet into the reaction flask. The oil bath was heated to a temperature of 50°C, then 10.4 g of 2-(3-chloropropyl)-2-methyl-1,3-dioxolane (Formula 2) was slowly added dropwise, and the temperature was raised to reflux for 2 hours after the drop was completed. Then the reaction solution was cooled to an internal temperature of 20°C, and 13.12 g of farnesyl acetone (Formula 4) was added dropwise. After the dropwise addition, the reaction was incubated for 3 hours, then the reaction solution was poured into ice cubes and stirred, and 10 ml of hydrochloric acid was added and stirred for 1 hour. , adding 50 ml of n-hexane for extraction, static layering, washing the organic layer with sodium bicarbonate, washing with saturated brine until neutral, and then evaporating the organic layer of n-h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com