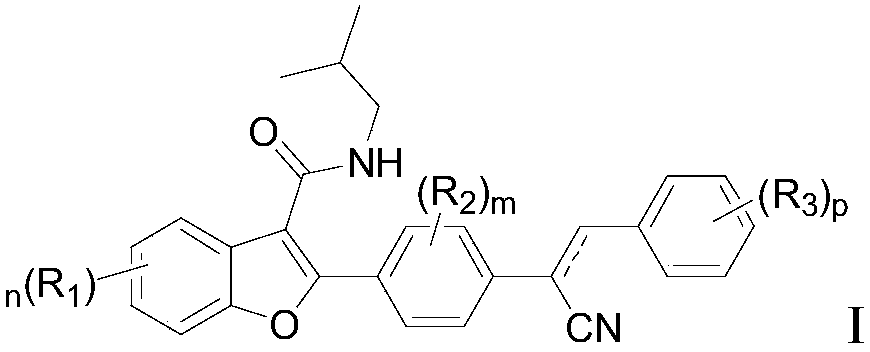
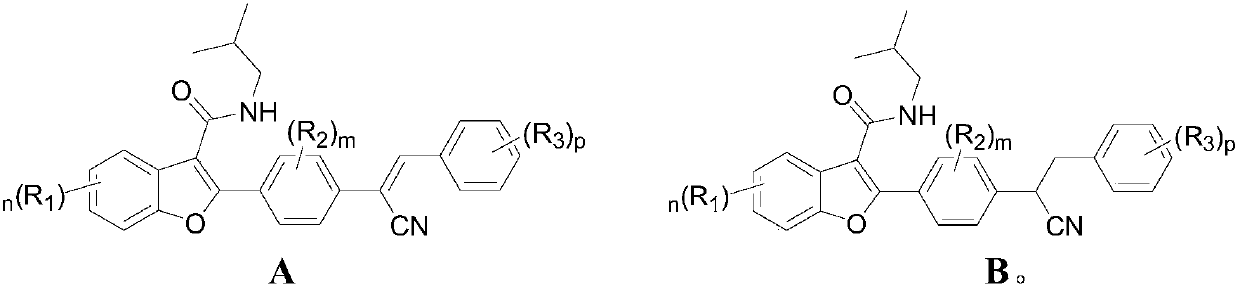
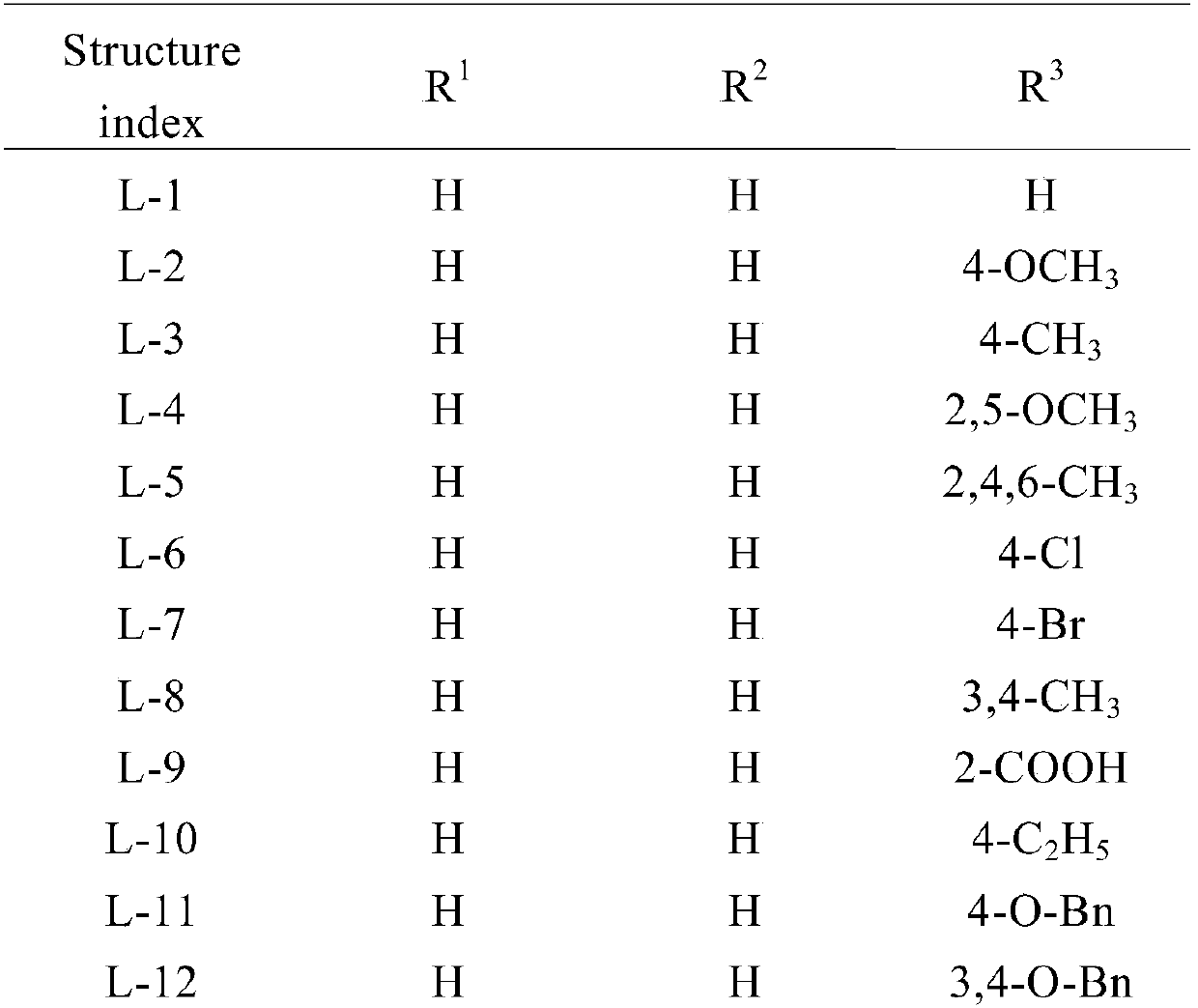
Preparation and application of 2-(4-substituted phenyl)-3-formamide benzofuranene cyanide compound
A technology of benzofurenyl cyanides and compounds, which is applied in the field of benzofurenyl cyanides and their preparation, and can solve the problems of lack of small molecule drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0073] Preparation of Benzofuryl Cyanides
[0074] The present invention also provides the preparation method of above-mentioned benzofuralene cyanide compound, and its schematic route comprises the steps:
[0075]
[0076] (a) get 1 molar equivalent of 2-hydroxybenzaldehyde and 1 molar equivalent of triphenyl (4-methylbenzyl) phosphine chloride in acetonitrile to reflux for 12 hours to obtain (E)-2-(4-methyl styryl) phenol.
[0077] (b) get 1 molar equivalent of (E)-2-(4-methylstyryl) phenol and 6 molar equivalents of potassium carbonate and 6 molar equivalents of iodine in tetrahydrofuran at room temperature for 1 hour to obtain 2-(4 -methylphenyl)benzofuran.
[0078] (c) Take 1 molar equivalent of 2-(4-methylphenyl)benzofuran, 8 molar equivalents of phosphorus oxychloride and 8 molar equivalents of N.N-dimethylformamide in 1,2-dichloroethane Heated under reflux in alkanes for 12 hours to obtain 2-(4-methylphenyl)benzofuran-3-aldehyde.
[0079] (d) Take 1 molar equiva...
Embodiment 1
[0111] Synthesis of E-2-(4-methylstyryl)phenol (Ⅰ)
[0112] Dissolve compound XII (100g, 248.2mmol) and 2-hydroxybenzaldehyde in 350mL of acetonitrile, stir at room temperature and slowly add DBU (91.3mL, 595.7mmol) dropwise, after the dropwise addition, heat and stir under reflux for 12h to complete the reaction, and cool to Concentrate the reaction solution at room temperature, neutralize with dilute hydrochloric acid to neutrality, add 200mL of water and extract 3 times with ethyl acetate, combine the organic phases, wash with 200mL saturated brine, dry over anhydrous sodium sulfate, concentrate the organic layer, and separate by silica gel chromatography Purified white solid powder I (31.8 g, yield=61%). 1 H NMR (400MHz, DMSO-d 6 )9.67(s,1H),7.52(d,J=7.43Hz,1H),7.40(d,J=7.83Hz,2H),7.32(d,J=16.82Hz,1H),7.09-7.17(m, 3H), 7.01-7.08(m, 1H), 6.83(d, J=7.83Hz, 1H), 6.77(t, J=7.43Hz, 1H), 2.27(s, 3H)
Embodiment 2
[0114] Synthesis of 2-(4-methylphenyl)benzofuran(Ⅱ)
[0115] Compound I (5.4g, 25.7mmol) and anhydrous K 2 CO 3 (21.2g, 154.2mmol) was dissolved in 150mL of tetrahydrofuran, and after stirring at room temperature for 1 hour, elemental iodine powder (39.4g, 154.2 mmol) was added in one go. A saturated aqueous solution of sodium sulfite was used to remove unreacted iodine, extracted three times with ethyl acetate, the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated organic layer, separated and purified by silica gel column chromatography to obtain white powder compound II ( 5.03 g, yield=94%). 1 H NMR (400MHz, DMSO-d 6 )7.78(d, J=8.22Hz, 2H), 7.57(d, J=8.61Hz, 1H), 7.60(d, J=7.43Hz, 1H), 7.32(s, 1H), 7.28(d, J= 8.22 Hz, 2H), 7.24 (dd, J = 1.17, 6.26 Hz, 1H), 7.19-7.22 (m, 1H), 2.32 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com