Culture system and method for amplifying hemopoietic stem cells as well as application thereof
A technology of hematopoietic stem cells and medium, applied in the direction of cell culture active agent, blood/immune system cells, tissue culture, etc., to achieve the effect of large quantity, low expansion rate and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The preparation of the umbilical cord blood hematopoietic stem cells includes: diluting the umbilical cord blood 2 to 3 times with normal saline, adding lymphocyte separation medium, centrifuging at 1500 to 2000 rpm / min for 20 minutes to obtain mononuclear cell layer (PBMC), washing with normal saline and resuspending Obtain PBMC cell aggregates; then separate CD34+ cells by magnetic beads method.
[0041] StemSpanSFEM II is a serum-free medium, the manufacturer is StemCell Technologies, the product number is 09655;
[0042] Recombinant human stem cell factor rhSCF (recombined human stem cell factor), the manufacturer is Stemimmune LLC, the product number is HHM-SF-1000;
[0043] Recombinant human thrombopoietin rhTPO (recombined human thrombopoietin), the manufacturer is Stemimmune LLC, the product number is HHM-TP-0100;
[0044] Recombinant human FMS-like tyrosine kinase 3 ligand rhFLT3L (recombined human FMS-liketyrosinekinase 3ligand), the manufacturer is Stemimmun...
Embodiment 1
[0060] 1. Obtain umbilical cord blood mononuclear cells;
[0061] (1) Dilute the umbilical cord blood with normal saline for 2 to 3 times, mix well and add dropwise to 0.4 times the volume of lymphocyte separation solution, taking care not to damage the interface;
[0062] (2) Use 1500-2000rpm / min to centrifuge for 20min. Due to different densities, the centrifuge tube is divided into four layers from top to bottom: the first layer is the plasma layer, the second layer is the ring-shaped milky white mononuclear cell layer (PBMC), and the second layer is the plasma layer. The third layer is the transparent separation liquid layer, and the fourth layer is the red blood cell layer;
[0063] (3) Use a straw to carefully draw the second layer of milky white mononuclear cell layer (PBMC) into another 50ml centrifuge tube, add physiological saline, and centrifuge again at 1500-2000rpm / min for 5-10min;
[0064] (4) Discard the supernatant and add physiological saline to resuspend, fi...
Embodiment 2
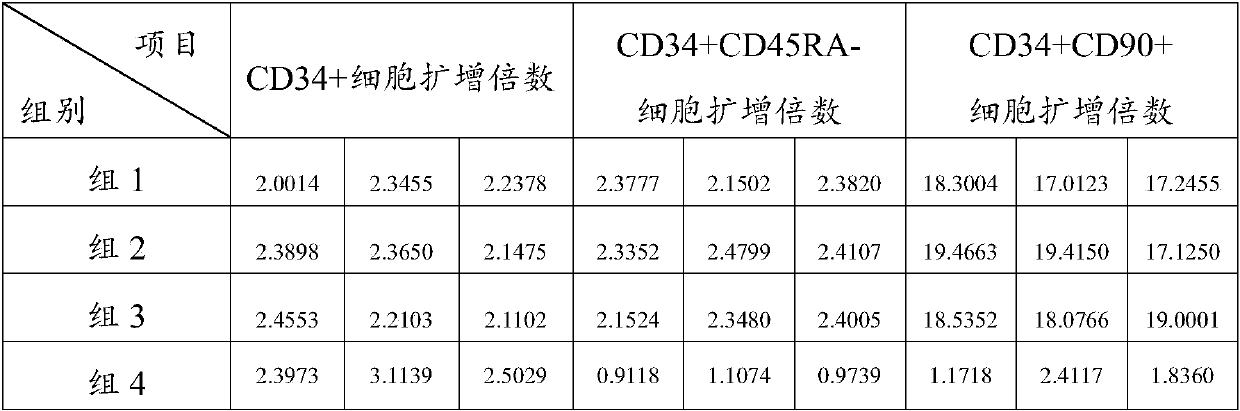
[0074] The contents of factors in the medium of each group are shown in Table 1:
[0075] Table 1 The content of factors in the medium of each group
[0076]
MS275
SCF
TPO
FLT3
group 1
1μM
80ng / ml
30ng / ml
90ng / ml
group 2
1μM
100ng / ml
50ng / ml
100ng / ml
group 3
1μM
120ng / ml
70ng / ml
110ng / ml
group 4
0
80ng / ml
30ng / ml
90ng / ml
[0077] Each substance was added to StemSpan SFEM II serum-free medium at the concentrations shown in Table 1.
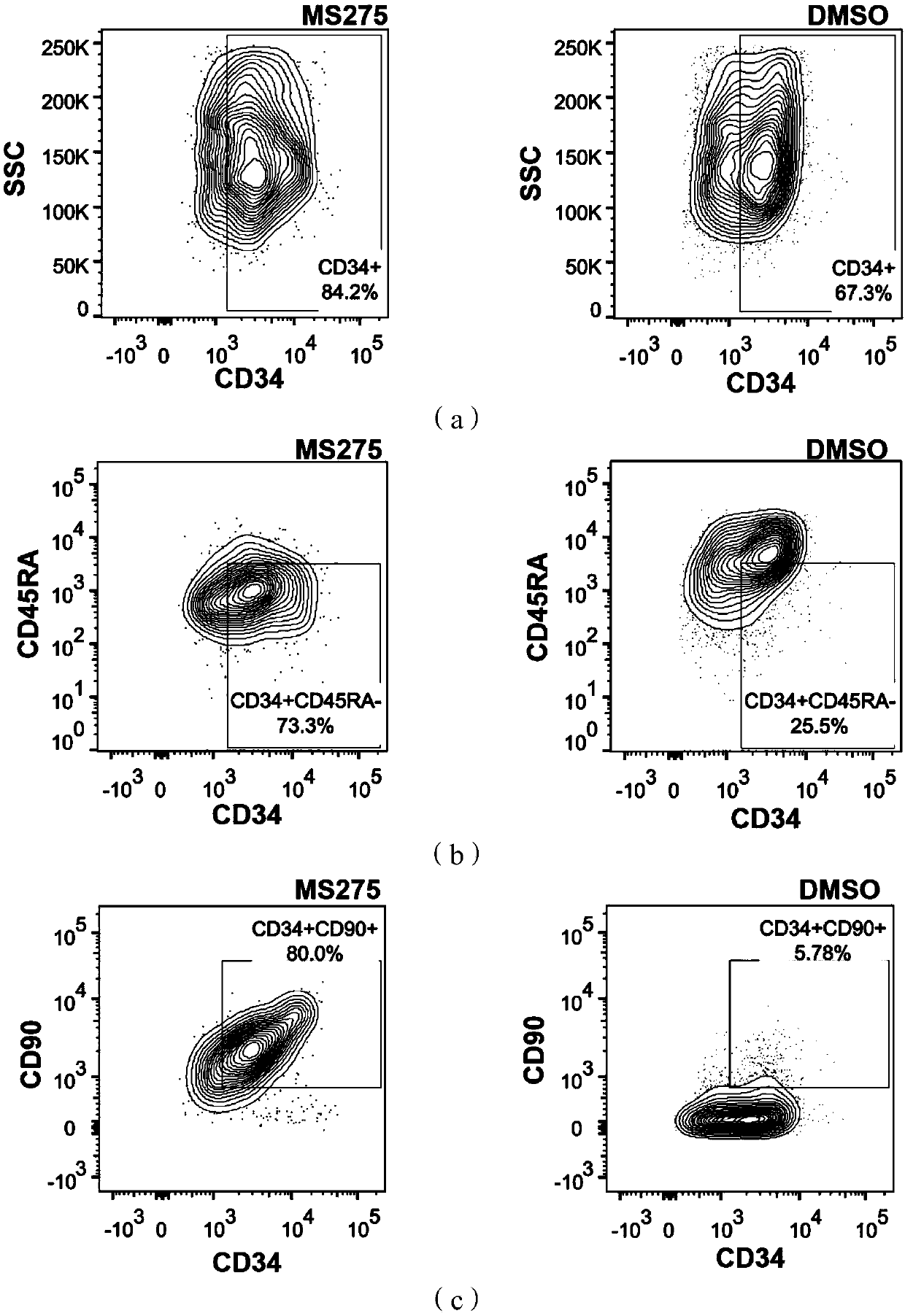
[0078] The CD34+ umbilical cord blood hematopoietic stem cells prepared in Example 1 were suspended and inoculated in the cell culture medium of each group for culture. Cells were seeded at a density of 1 × 10 in a 24-well plate 4 cells / well, placed at 37°C, 5% CO 2 Incubator cultivation. According to the state of cell culture, 500 μL of fresh cell culture medium of each group was added every 2 days, and a large number of hem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com