Use of quassin compounds in preparation of anti-complement drug
A compound, picarinnin technology, which is applied in the preparation of sugar derivatives, drug combinations, antipyretics, etc., can solve the problems such as the separation of compounds with complement inhibitory effect that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
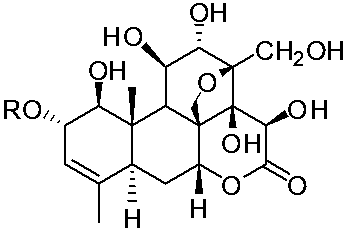
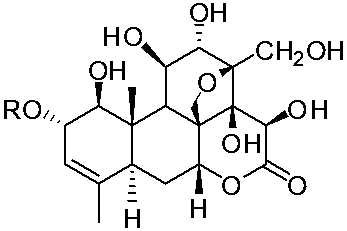
[0025] Example 1. Activity-directed separation and preparation of picrin compounds with anti-complement activity:
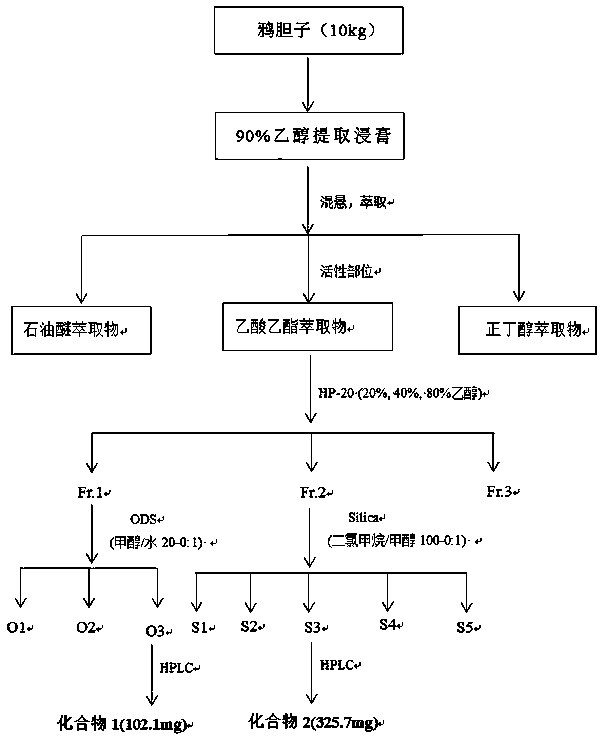
[0026] according to figure 1 The flow chart in the activity-guided separation of bittern compounds with anti-complement activity from B. javanica, the specific method is as follows:
[0027] Take 10kg of dried fruit of Brucea javanica, degrease it with petroleum ether, and extract it twice with ethanol with a volume fraction of 90%. , to obtain 15L concentrated solution, then use ethyl acetate to extract the concentrated solution, the extract is concentrated and dried to obtain 350g ethyl acetate extract; get ethyl acetate extract and adopt macroporous adsorption resin HP-20 to separate and refine, use Volume fractions of 20%, 40%, and 80% ethanol were eluted sequentially to obtain the main active site 40% ethanol elution fraction Fr.1 (182g) and 80% ethanol elution fraction Fr.2 (114g);
[0028] Fraction Fr.1 was separated by ODS column chromatography, and gr...
Embodiment 2
[0030] Example 2. Anti-complement classical pathway test in vitro:
[0031] (1) Experimental principle: Complement can cause hemolysis of sheep red blood cells (SRBC) sensitized by hemolysin (anti-sheep red blood cell antibody). When the concentration of sensitized sheep red blood cells is constant, the degree of hemolysis is proportional to the content and activity of complement. Therefore, after different dilutions of fresh serum to be tested, it reacts with sensitized red blood cells to determine the degree of hemolysis. The minimum serum volume at 50% hemolysis is used to determine the end point, and the total hemolytic activity of complement can be measured.
[0032] The 50% hemolysis test is to obtain the minimum amount of serum that can hemolyze 50% of the sensitized sheep red blood cells, and then calculate the complement content per milliliter of serum. Taking the amount of complement as the abscissa and the percentage of hemolysis as the ordinate, a clear "S"-shaped ...
Embodiment 3
[0037] Example 3. Anti-complement alternative pathway test in vitro:
[0038] (1) Experimental principle: Rabbit erythrocytes can activate the human complement alternative pathway without sensitization, leading to lysis of rabbit erythrocytes. Adding ethylene glycol-bis-aminotetracetate (EGTA) to the reaction system can chelate plasma Ca2+, and the binding ability of EGTA to Mg2+ is very weak, so the classical pathway is blocked. According to the degree of hemolysis of rabbit erythrocytes, the total complement activity of the alternative complement pathway can be determined.
[0039] (2) The specific experimental process is: Take 0.2 ml of complement (human serum), add AP diluent (barbital buffer, pH=7.4, containing 5mM Mg2+, 8mM EGTA) to prepare a 1:1 solution, and double Dilute to 1:2, 1:4, 1:8, 1:16, 1:32, 1:64 and 1:128 solutions. Take 0.15ml of complement of each concentration, 0.15ml of AP diluent and 0.20ml of rabbit erythrocyte suspension (0.5%RE) with a volume fract...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com