Method for preparing titanium dioxide and iron oxide by using fluoride purifying titanium-iron material
A technology of titanium dioxide and ferro-titanium, which is applied in the field of preparation of titanium dioxide and iron oxide, can solve the problem of low quality of titanium-containing elements, and achieve the effects of effective control of product quality, low cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
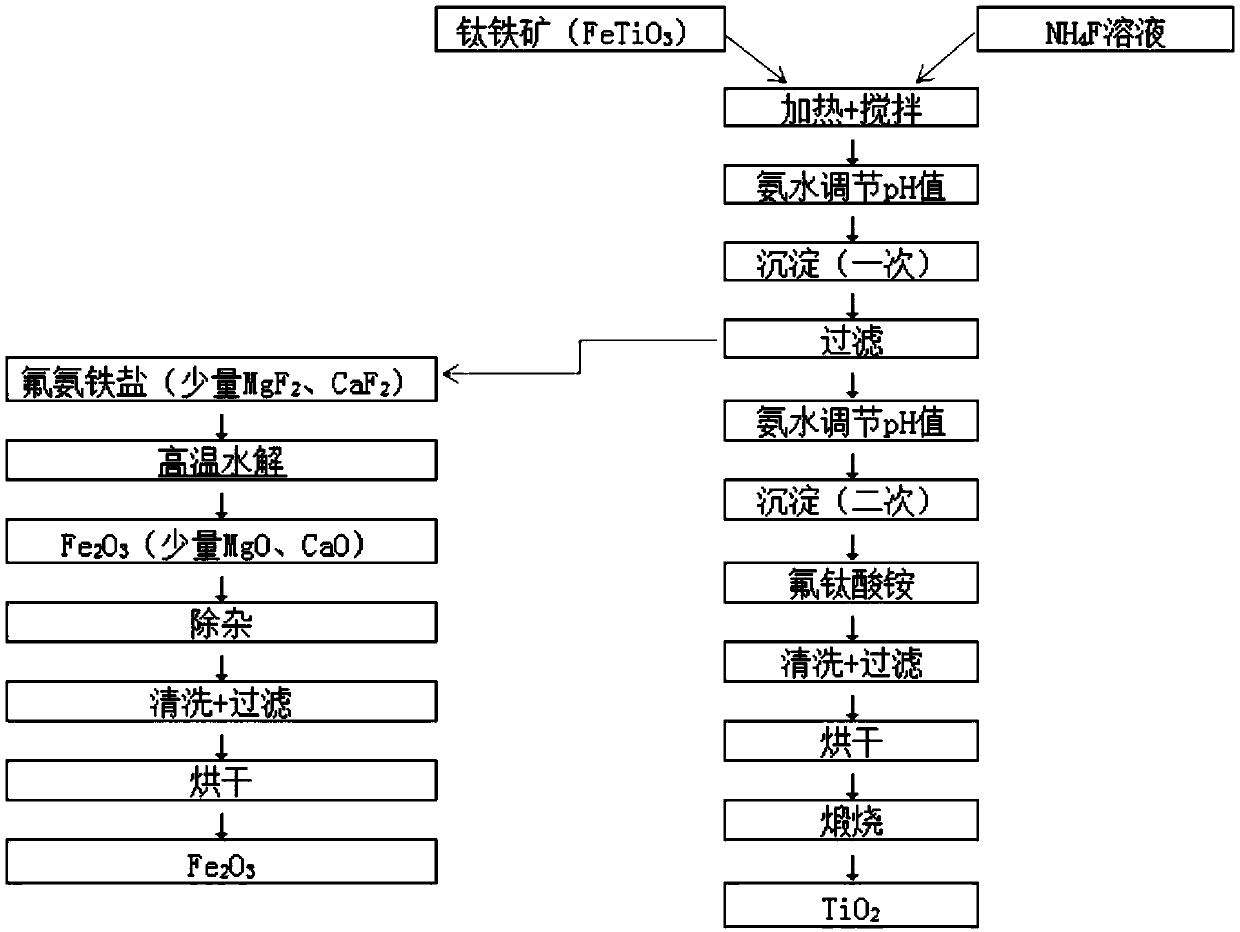
[0062] The composition of ilmenite is: FeTiO 3 90wt%; CaO 1wt%; MgO 4wt%, the rest is impurities.
[0063] Mix ammonium fluoride aqueous solution with a mass concentration of 40% and ilmenite at a solid-to-liquid ratio of 0.5:1 at 80 rpm and then heat at 50°C for 0.5 hours to obtain a mixture;
[0064] Using ammonia water to adjust the pH value of the mixture to 6.5 to obtain the first precipitate;
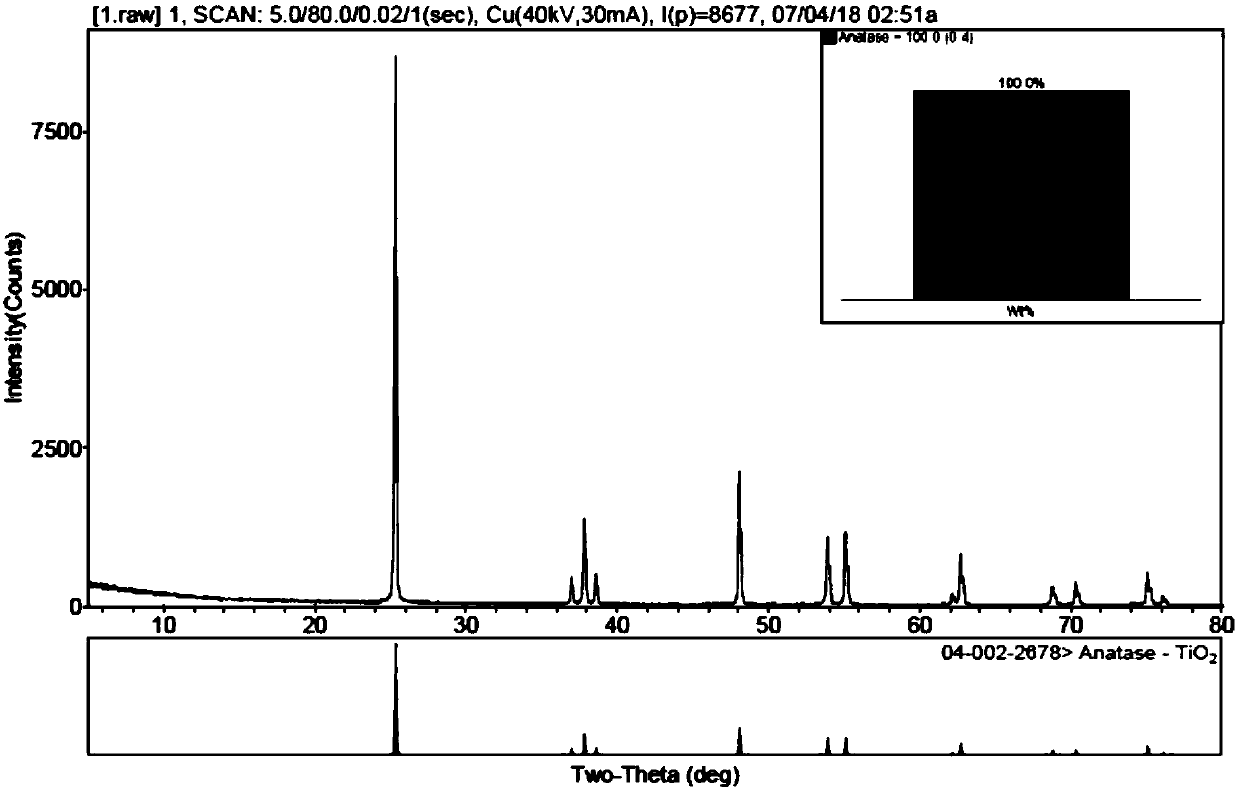
[0065] The first precipitate is separated by pressing and filtering with a membrane filter, and the pH value of the remaining solution is adjusted to 8.5 with ammonia water to obtain a second precipitate, which is separated by pressing and filtering with a membrane filter.
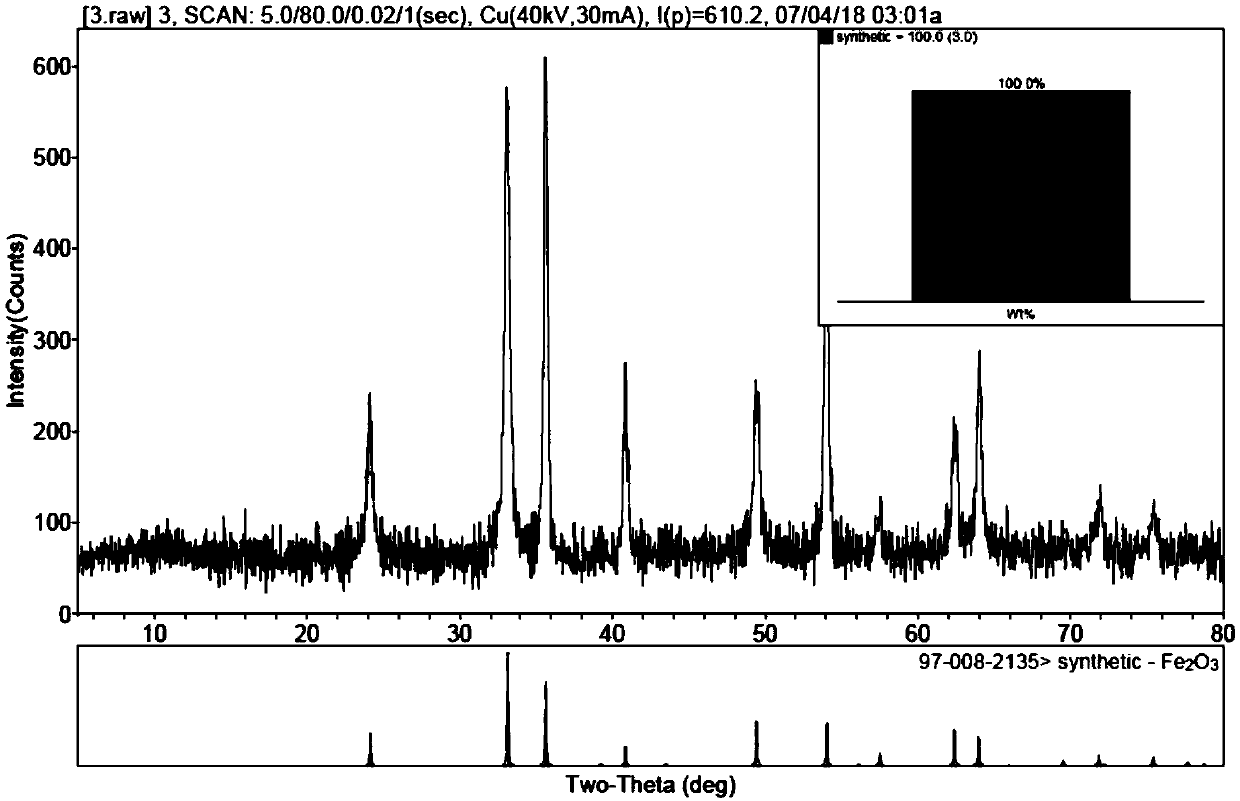
[0066] The first precipitate is hydrolyzed at 500°C at high temperature, and the product after high temperature hydrolysis is mixed with ammonium stearate for impurity removal. Ammonium stearate absorbs Ca and Mg ions and floats on the upper layer to form a suspended solid. Pour the upper layer solution to r...
Embodiment 2
[0073] The composition of ilmenite is: FeTiO 3 92wt%; CaO 2wt%; MgO 4wt%, and the balance is impurities.
[0074] Mix ammonium fluoride aqueous solution with a mass concentration of 50% and ilmenite at a solid-to-liquid ratio of 1:1 at 100 rpm, and then heat at 75°C for 1.5 hours to obtain a mixture;
[0075] Using ammonia water to adjust the pH value of the mixture to 7 to obtain the first precipitate;
[0076] The first precipitate is separated by pressing and filtering with a membrane filter, the pH of the remaining solution is adjusted to 9 with ammonia water, and the second precipitate is separated by pressing and filtering with a membrane filter.
[0077] The first precipitate was hydrolyzed at 700°C at high temperature, and the product after high temperature hydrolysis was mixed with ammonium stearate to remove impurities. Ammonium stearate adsorbed Ca and Mg ions and floated on the upper layer to form a suspended solid, and poured the upper layer solution to remove t...
Embodiment 3
[0083] The composition of ilmenite is: FeTiO 3 94wt%; CaO 3wt%; MgO 2wt%, the balance is impurities.
[0084] Mix ammonium fluoride aqueous solution with a mass concentration of 60% and ilmenite at a solid-to-liquid ratio of 2:1 at 120 rpm, and then heat at 100°C for 4 hours to obtain a mixture;
[0085] Using ammonia water to adjust the pH value of the mixture to 7.5 to obtain the first precipitate;
[0086] The first precipitate is separated by pressing and filtering with a membrane filter, the pH of the remaining solution is adjusted to 9.5 with ammonia water, and the second precipitate is separated by pressing and filtering with a membrane filter.
[0087] The first precipitate is hydrolyzed at 900°C at high temperature, and the product after high temperature hydrolysis is mixed with ammonium stearate for impurity removal. Ammonium stearate absorbs Ca and Mg ions and floats on the upper layer to form a suspension, and the upper layer solution is poured to remove the susp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com