Application of polypeptide for preparing medicine for treating Alzheimer disease
A technology for Alzheimer's disease and uses, which is applied in the field of peptides used to prepare medicines for the prevention and treatment of Alzheimer's disease, and can solve problems such as memory loss, apraxia, and inability to take care of themselves in life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
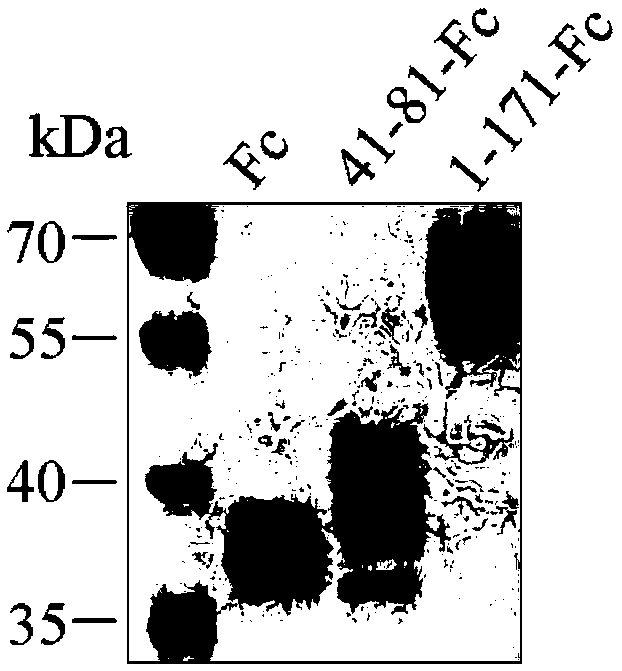
[0031] Example 1: Preparation of sTREM2 41-81-Fc and sTREM2 1-171-Fc fusion proteins
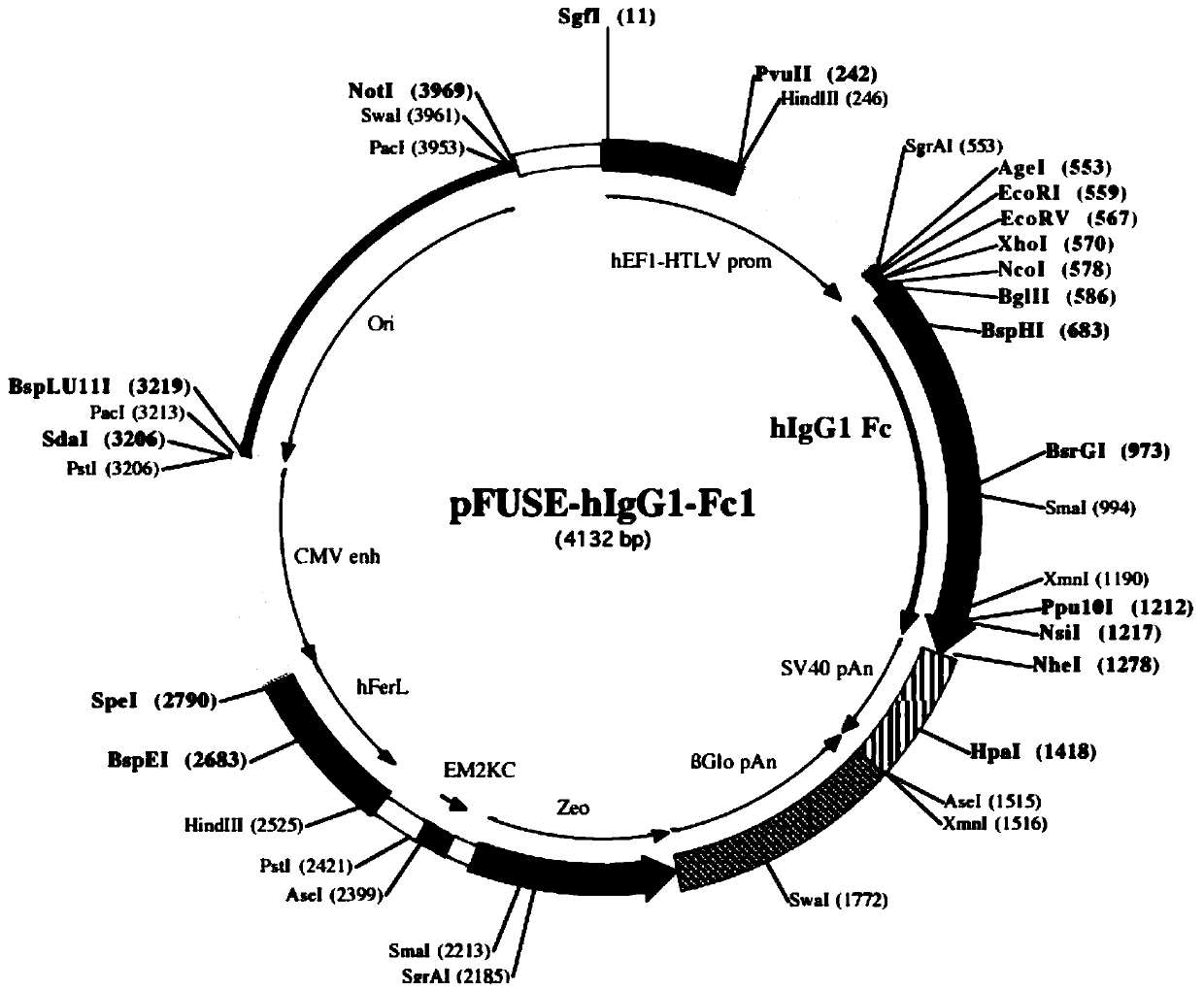
[0032] Construction of pFUSE-sTREM2 1-171-hIgG1-Fc1 expression plasmid: use pFUSE-hIgG1-Fc1 (purchased from Invivogen, product number is pfuse-hg1fc1, see vector picture figure 1 ) is a eukaryotic expression vector, EcoRI and XhoI restriction sites are selected as the cloning site, and pFUSE-sTREM2 1-171-hIgG1-Fc1 expression plasmid is constructed. The steps are as follows: Design upstream primer: 5'-CG GAATTC ATGGAGCCTCTCCGGCTGC-3'SEQ ID NO: 3, where the underline is the EcoRI restriction site; downstream primer: 5'-CCG CTCGAG TTTGGGAAGGGGATTTCTC-3' SEQ ID NO: 4, where the underline is the XhoI restriction site.
[0033] The nucleotide sequence of TREM2 is (purchased from Beijing Yiqiao Sino Biotechnology Co., Ltd., article number HG11084-M: TREM-2 cDNA ORF Clone in Cloning Vector, Human):
[0034]
[0035]
[0036] The single underline is the signal peptide sequence of the prote...
Embodiment 2
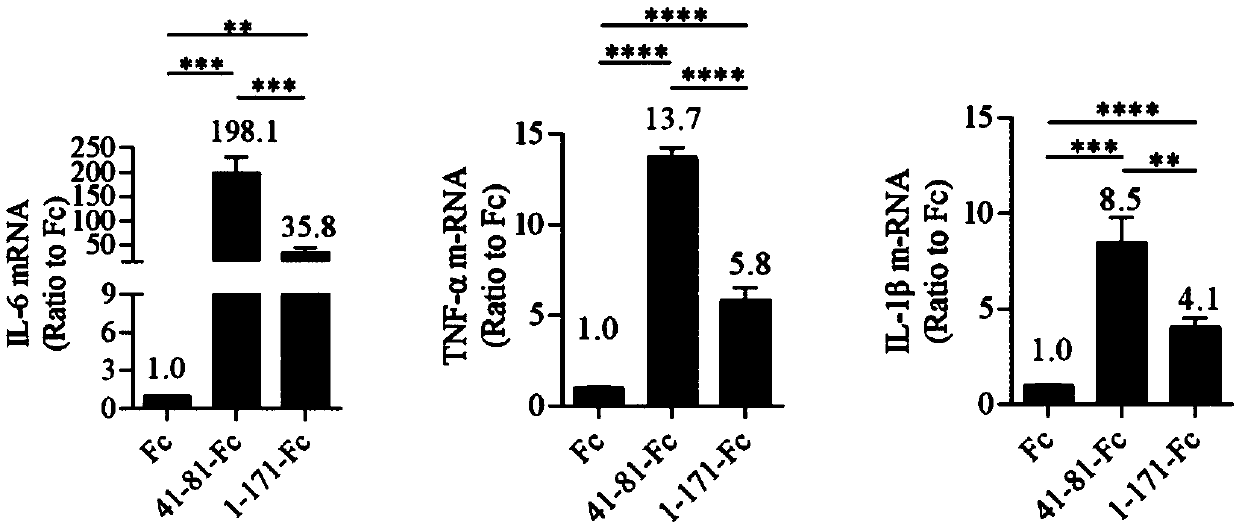
[0041] Example 2: Effects of sTREM2 41-81-Fc and sTREM2 1-171-Fc fusion proteins on the inflammatory response of microglial cells
[0042] Take 5×10 5 Cell number Primary microglial cells (isolated from the brain of C57BL / 6N neonatal mice at 3-4 days after birth) were cultured in 12-well plates. After 24 hours, the cells were treated with 20nM sTREM2 41-81-Fc fusion protein, sTREM21-171-Fc fusion protein (i.e. the protein obtained in Example 1) and Fc control protein (i.e. pFUSE-hIgG1-Fc1 vector) Culture was continued for 4 hours in serum-free medium. Immediately place the cells on ice and wash 3 times with pre-cooled 1×PBS, use TRIzol reagent (Thermo Fisher, catalog number 15596018) to extract total RNA, and reverse transcription kit (Beijing Quanshijin, catalog number AT314-02) recorded as cDNA. The mRNA levels of IL-6, TNF-α, IL-1β, and β-Actin were detected by real-time quantitative PCR (RT-qPCR), and the levels of the other three inflammatory factors were analyzed usin...
Embodiment 3
[0043] Example 3 Effects of sTREM2 41-81-Fc and sTREM2 1-171-Fc fusion proteins on microglia apoptosis
[0044] to 10 5 Cell number The primary microglial cells were plated on glass slides in a 24-well plate (coated with Poly-Lysine for 24 hours in advance) for culture. After 48 hours, the cells were treated with 20nM sTREM2 41-80-Fc fusion protein, sTREM2 1-171-Fc fusion protein (i.e. protein obtained in Example 1) and Fc control protein (i.e. pFUSE-hIgG1-Fc1 vector) respectively and placed in Culture was continued for 24 hours in serum-free medium. The cells were placed on ice and washed three times with pre-cooled 1×PBS, fixed with 4% paraformaldehyde at room temperature for 20 min, and permeabilized with 0.2% Triton X-100 for 5 min. Subsequently, apoptotic cells were detected using the terminal deoxynucleotidyl transferase-mediated dUTP method for nick end labeling (TUNEL) detection kit (Promega, G3250) and nuclear staining with nuclear dye DAPI to analyze the total cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com