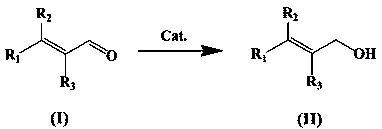
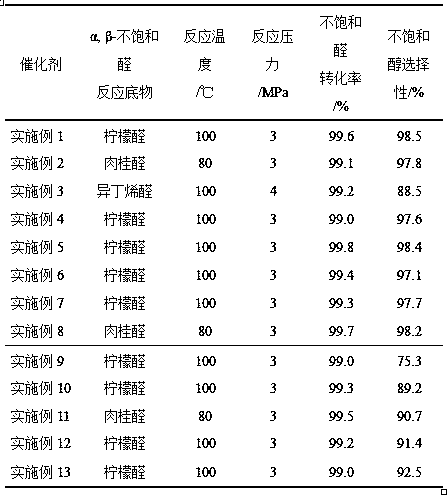
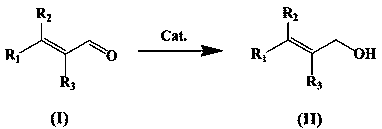
Catalyst for selective hydrogenation of alpha, beta-unsaturated aldehyde to prepare unsaturated alcohol, preparation method and application thereof
An aldehyde-selective and unsaturated technology, applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, preparation of hydroxyl compounds, etc., can solve the problems of organic solvent pollution and low selectivity of unsaturated alcohols , to achieve the effect of eliminating pollution, high selectivity and reducing burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation method of a catalyst for selective hydrogenation of α, β-unsaturated aldehydes to unsaturated alcohols
[0047] Include the following steps:
[0048] (1) Preparation of carrier
[0049] Add 8g of 3% aqueous sodium bicarbonate solution dropwise to 378.5g of 10% cerium nitrate solution, leave the resulting suspension to age at room temperature for 8 hours, filter out the filter cake with suction, dry at 120°C for 12 hours, and roast at 500°C 4h, get CeO 2 carrier.
[0050] (2) Preparation of catalyst precursor
[0051] An impregnating solution containing ruthenium chloride, ferric chloride and zinc acetate was prepared, and the mass contents of ruthenium, iron and zinc in the impregnating solution were 3.0%, 5.0% and 0.5% respectively. Immerse 10g of the carrier in 10g of impregnating solution, stir and adsorb at room temperature for 24h, air-dry naturally, and then dry at 60°C for 12h to prepare a catalyst precursor. In the prepared catalyst precursor, the ...
Embodiment 2
[0057] Preparation method of a catalyst for selective hydrogenation of α, β-unsaturated aldehydes to unsaturated alcohols
[0058] Include the following steps:
[0059] (1) Preparation of carrier
[0060] Add 8g of 3% aqueous sodium bicarbonate solution dropwise to 378.5g of 10% cerium nitrate solution, leave the resulting suspension to age at room temperature for 8 hours, filter out the filter cake with suction, dry at 120°C for 12 hours, and roast at 500°C 4h, get CeO 2 carrier.
[0061] (2) Preparation of catalyst precursor
[0062] An impregnation solution containing ruthenium nitrate, iron nitrate and zinc nitrate was prepared, and the mass contents of ruthenium, iron and zinc in the impregnation solution were 5.0%, 1.5% and 1.0% respectively. Immerse 10g of the carrier in 10g of impregnating solution, stir and adsorb at room temperature for 24h, air-dry naturally, and then dry at 60°C for 6h to obtain a catalyst precursor. In the prepared catalyst precursor, the Ru l...
Embodiment 3
[0068] Preparation method of a catalyst for selective hydrogenation of α, β-unsaturated aldehydes to unsaturated alcohols
[0069] Include the following steps:
[0070] (1) Preparation of carrier
[0071] Add 8g of 3% aqueous sodium bicarbonate solution dropwise to 378.5g of 10% cerium nitrate solution, leave the resulting suspension to age at room temperature for 8 hours, filter out the filter cake with suction, dry at 120°C for 12 hours, and roast at 500°C 2h, get CeO 2 carrier.
[0072] (2) Preparation of catalyst precursor
[0073] An impregnation solution containing ruthenium acetate, iron nitrate and zinc nitrate is prepared, and the mass contents of ruthenium, iron and zinc in the impregnation solution are 1.0%, 0.05% and 0.1% respectively. Immerse 10g of carrier in 10g of impregnating solution, stir and adsorb at room temperature for 24h, air-dry naturally, and then dry at 60°C for 12h to obtain a catalyst precursor. In the prepared catalyst precursor, the Ru loadi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com