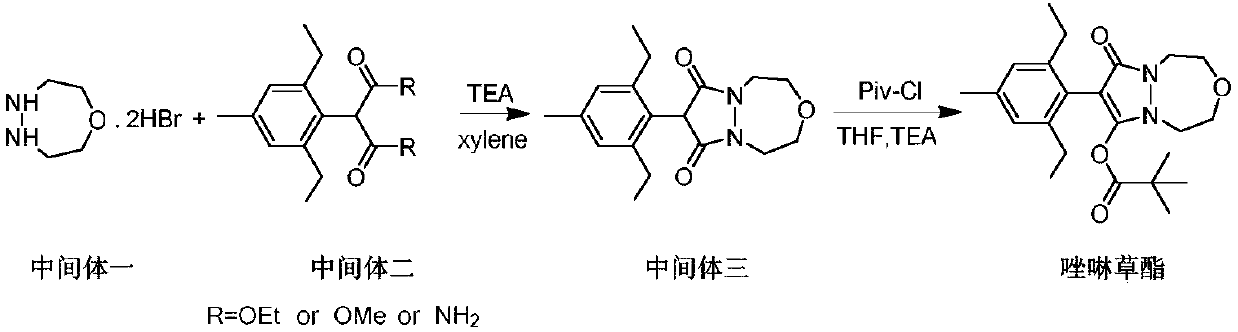
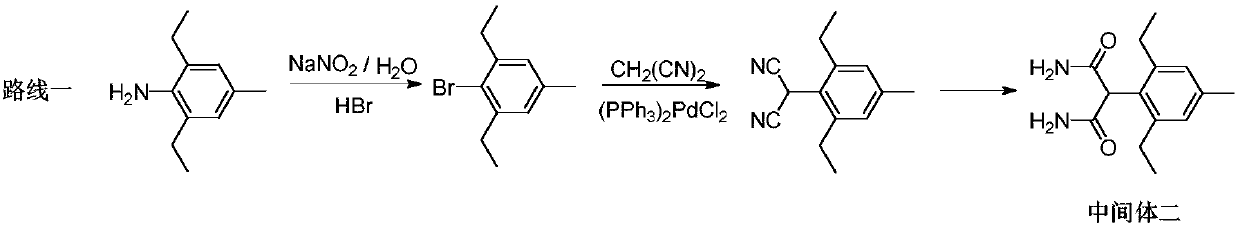
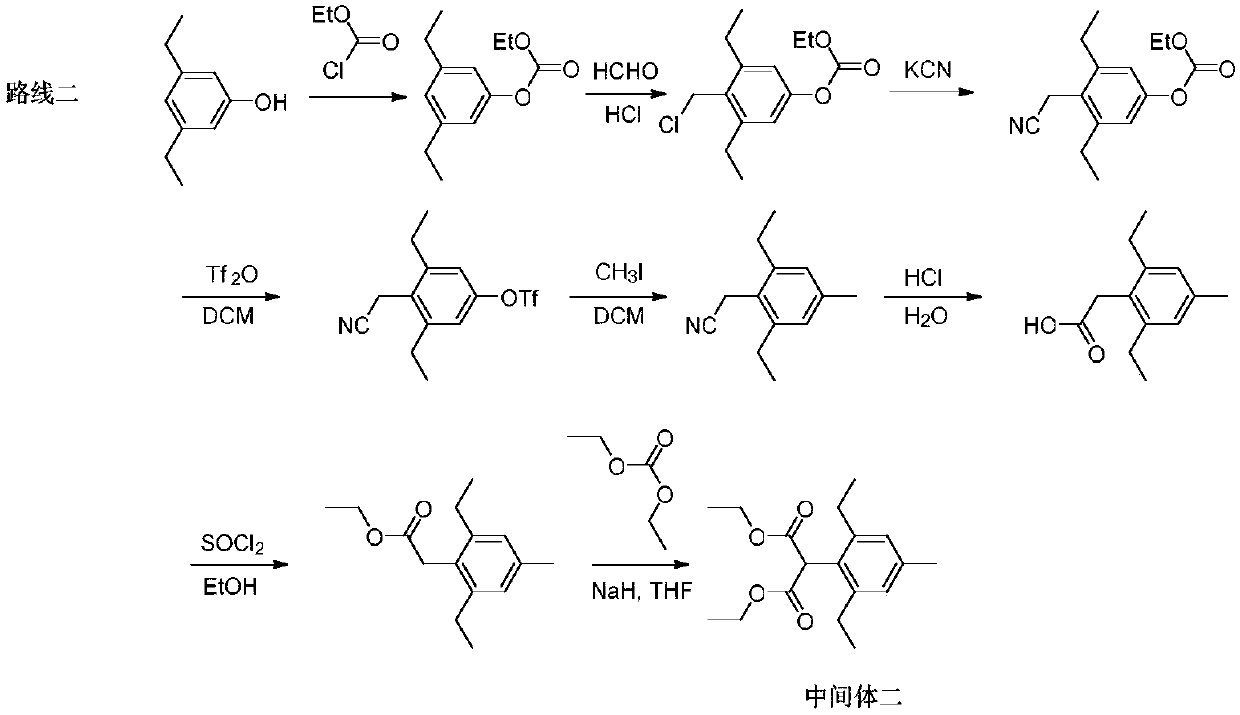
Synthesis method of pinoxaden intermediate (2, 6-diethyl-4-methyl)phenylacetic acid
A technology of pinoxaden and a synthesis method, applied in the field of synthesis of pinoxaden intermediate phenylacetic acid, can solve the problems of limited industrial application, high cost, expensive palladium catalyst, etc., and achieve high industrial value and yield High, the effect of avoiding the use of palladium catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Step 1: In a 250mL four-neck flask, add 65mL of 45% hydrobromic acid solution (0.36mol), slowly add 16.33g of 2,6-diethyl-4-methylaniline (molecular weight 163.3, 0.1mol) dropwise, drop Bi raised the temperature to 80°C and stirred for half an hour and then lowered to -10°C. Control the temperature at -10°C to -5°C, and slowly add sodium nitrite solution (8g of sodium nitrite dissolved in 35mL of water, 0.116mol) dropwise, and the dripping is completed in about 1 hour. After dropping, keep stirring at -10°C to -5°C for half an hour to form a diazonium salt solution. Add 13.9g of ferrous sulfate heptahydrate (molecular weight: 278, 0.05mol) and 65mL of 45% hydrobromic acid solution (0.36mol) into another four-neck flask, and heat to 80°C. The above diazonium salt solution was slowly added dropwise to another four-necked flask, and the drop was completed in about 1 hour. After the drop was completed, the temperature was kept stirring at 80° C. for 1 hour. Cool down to r...
Embodiment 2
[0044] Example 2: 2-bromoethanol example
[0045] Step 1: In a 250mL four-neck flask, add 45mL of 45% hydrobromic acid solution (0.25mol), slowly add 16.33g of 2,6-diethyl-4-methylaniline (molecular weight 163.3, 0.1mol) dropwise, drop Bi raised the temperature to 80°C and stirred for half an hour and then lowered to -10°C. Control the temperature at -10°C to -5°C, and slowly add sodium nitrite solution (8g of sodium nitrite dissolved in 35mL of water, 0.116mol) dropwise, and the dripping is completed in about 1 hour. After dropping, keep stirring at -10°C to -5°C for half an hour to form a diazonium salt solution. Add 11.1 g of ferrous sulfate heptahydrate (molecular weight: 278, 0.04 mol) and 54 mL of 45% hydrobromic acid solution (0.30 mol) into another four-neck flask, and heat to 80°C. The above diazonium salt solution was slowly added dropwise to another four-necked flask, and the drop was completed in about 1 hour. After the drop was completed, the temperature was kep...
Embodiment 3
[0048] Example 3: Example of sec-butyllithium
[0049] Step 1: In a 250mL four-neck flask, add 72mL of 45% hydrobromic acid solution (0.40mol), slowly add 16.33g of 2,6-diethyl-4-methylaniline (molecular weight 163.3, 0.1mol) dropwise, drop Bi raised the temperature to 80°C and stirred for half an hour and then lowered to -10°C. Control the temperature at -10°C to -5°C, and slowly add sodium nitrite solution (8g of sodium nitrite dissolved in 35mL of water, 0.116mol) dropwise, and the dripping is completed in about 1 hour. After dropping, keep stirring at -10°C to -5°C for half an hour to form a diazonium salt solution. Add 27.8g of ferrous sulfate heptahydrate (molecular weight: 278, 0.1mol) and 90mL of 45% hydrobromic acid solution (0.50mol) into another four-neck flask, and heat to 80°C. The above diazonium salt solution was slowly added dropwise to another four-necked flask, and the drop was completed in about 1 hour. After the drop was completed, the temperature was kep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com