Recombinant acetylated lysine arginine N-terminal protease and preparing method and applications thereof
A technology for acetylating lysine-arginine and lysine-arginine, which is applied in the field of protease, can solve the problems of increasing the stability of protease at the N-terminal of lysine-arginine, and achieve high stability and high enzyme activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 [Construction of recombinant lysine arginine N-terminal protease Escherichia coli expression vector]
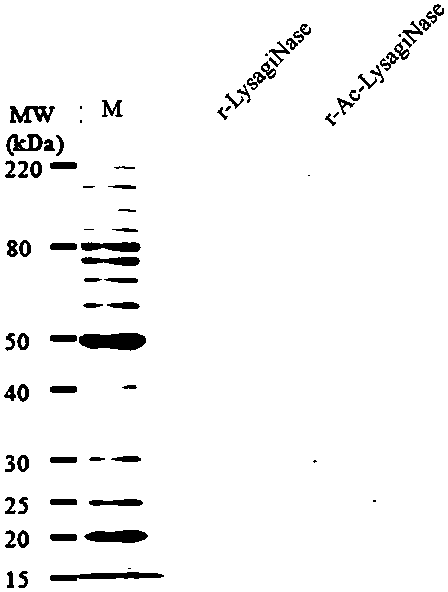
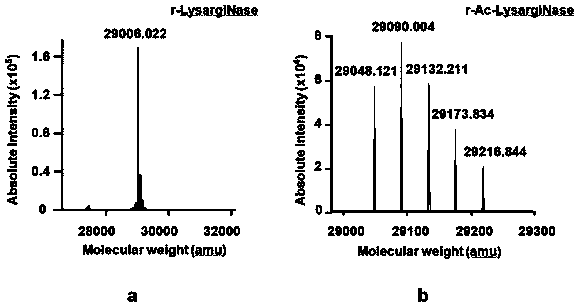
[0062] The recombinant lysine-arginine N-terminal protease gene sequence of the present invention is derived from Methanosarcina acetivorans (GenBank No. AE010299), and the gene sequence is shown in SEQ ID NO.2. We introduced a 6×His tag and an enterokinase cleavage site DDDDK at its N-terminus (a total of 15 amino acids were added) to obtain an open reading frame (ORF) encoding 357 amino acids, which is the sequence shown in SEQ ID NO.3, which corresponds to The protein sequence of the gene is shown in SEQ ID NO.4, and the gene coding sequence and corresponding protein sequence are also shown in figure 1 middle. Insert the sequence shown in SEQ ID NO.3 between the Nco I / Xho I restriction sites of the pET28a vector (Novagen, product number 69864-3CN) to obtain a recombinant lysine-arginine N-terminal protease recombinant expression vector, named pET -Lysarg...
Embodiment 2
[0064] Example 2 [Fermentation and Thalline Collection of BL21-LysargiNase Strain]
[0065] The recombinant strain BL21-LysargiNase was activated on a solid LB / Amp plate to obtain a single clone of the BL21-LysargiNase strain. A single clone of BL21-LysargiNase strain was inoculated in 50 mL of LB liquid medium (the concentration of ampicillin was 100 μg / mL), and cultured on a shaker at 37°C. When the OD of LB liquid culture system 600 When it is 1.5-2.0 (with LB liquid medium as blank control), the seed liquid of BL21-LysargiNase bacterial strain is obtained.
[0066] The seed liquid of the BL21-LysargiNase strain was inoculated separately into a 14L fermenter (BioFlo 310, New Brunswick Scientific Company) equipped with 5L high-density fermentation basal medium sterilized by 121°C damp heat and autoclave, and the culture system after inoculation OD 600 0.01 (with the high-density fermentation basal medium as the blank control). Initial fermentation parameters: the culture...
Embodiment 3
[0074] Embodiment 3 [purification of recombinant lysine arginine N-terminal protease]
[0075] Get the BL21-LysargiNase thalline that 200g embodiment 2 obtains and resuspend in 20mM Tris pH 7.5 according to the ratio of 1:10 (w / v), 150mM NaCl damping fluid, mix homogeneously with high-speed tissue shearing machine, high-pressure homogenate is broken (APV -1000 Homogenizer Breaker, SPX FLOW, Inc.). Set the pressure of the high-pressure homogenate to 800 bar, break continuously for 3 times and then centrifuge at 13,000 g at 4°C for 30 min. Collect the supernatant and perform metal-chelate affinity chromatography on AKTA Purifier (GE healthcare) (HisTrapFF 5 mL prepacked column, GE healthcare, Cat. No. 17-5255-01).
[0076] The chromatographic column was pre-equilibrated with buffer A (20mM Tris, 150mM NaCl, pH 7.5) before sample loading, and the sample loading flow rate was 5mL / min. After sample loading, use 10 column volumes of buffer A and 10 column volumes of 5% buffer solu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com