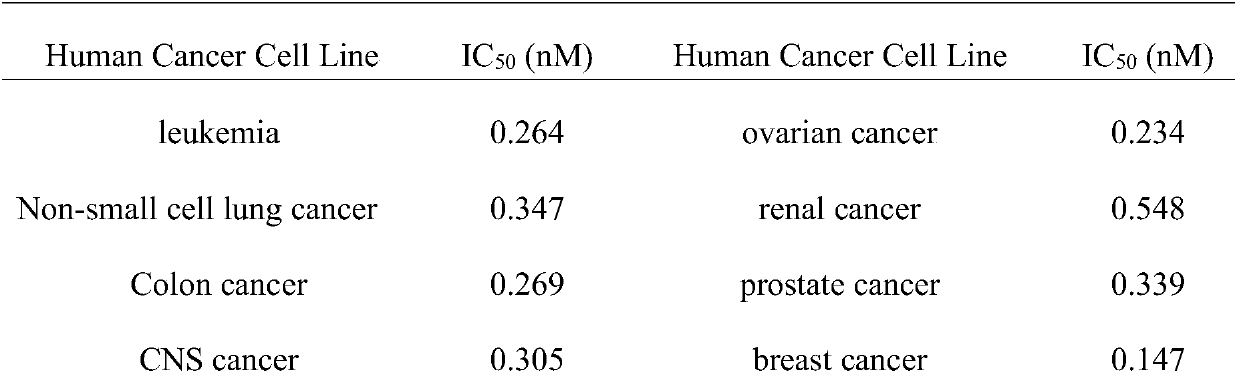
Preparation method for biological active peptide N14-Desacetoxytubulysin H
A technology of biologically active peptides and compounds, applied in the field of medicine, can solve the problems of unsatisfactory synthesis and imperfect synthetic route, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
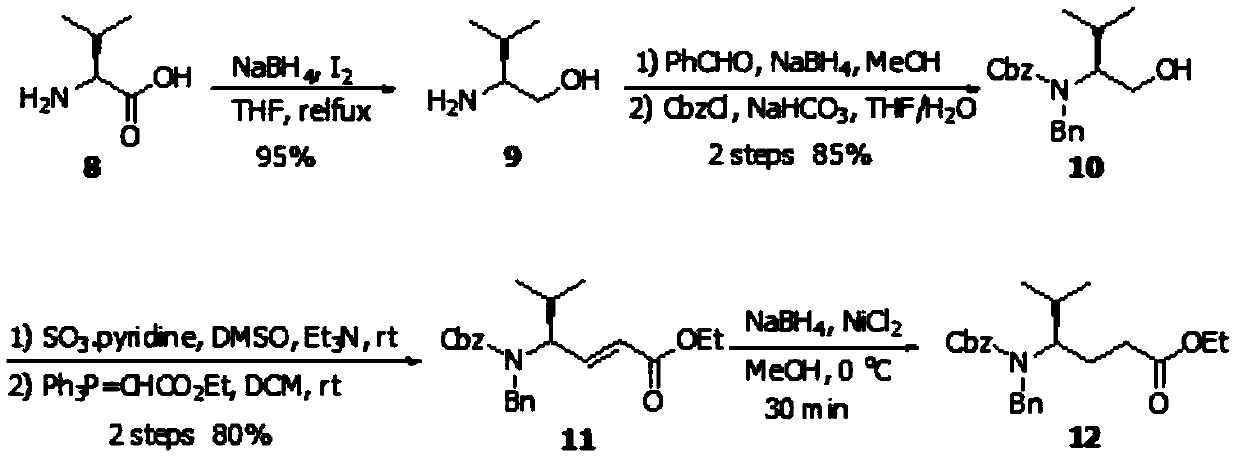
[0065] Embodiment 1 prepares TUV fragment (2):
[0066] The synthetic route of TUV fragment (2) is as follows:
[0067]
[0068]
[0069] Include the following steps:
[0070] Step 1, the synthesis of compound 10:
[0071] First, compound 9 was prepared according to the literature (Macromolecules, 2012, 45(20): 8172-8192);
[0072] Compound 9 (39g, 378.15mmol) was dissolved in methanol (500mL), benzaldehyde (39mL, 378.15mmol) was added, heated to reflux for 4h, cooled to 0°C in an ice-water bath, and sodium borohydride (43g, 1.13 mol), then stirred the reaction at room temperature for 12 h, concentrated under reduced pressure, added dilute hydrochloric acid to quench the reaction, and then basified the solution with aqueous sodium hydroxide to pH=10, extracted three times with dichloromethane (300 mL), combined the organic phases, Dry over anhydrous sodium sulfate and concentrate under reduced pressure;
[0073] Then add tetrahydrofuran / water (1:1, 600mL) mixed solve...
Embodiment 2
[0102] Example 2 Bioactive Peptide N 14 - Synthesis of Desacetoxytubulysin H:
[0103] Its synthetic route is as follows:
[0104]
[0105]
[0106] Include the following steps:
[0107] Step 1, the synthesis of compound 4:
[0108] Dissolve compound 2 (1g, 1.9mmol) in THF (60mL), add water (3mL) and triphenylphosphine (5.0g, 19mmol), heat to reflux for 3h, cool to room temperature, concentrate under reduced pressure, direct column chromatography Separation (mobile phase: methanol: dichloromethane 1: 20) separation and purification;
[0109] Add anhydrous dichloromethane (100mL), add compound 3 (0.87g, 3.8mmol), EDCI (1.82g, 9.5mmol) and HOBt (1.28g, 9.5mmol), stir well and drop triethylamine (2.6mL , 19mmol), stirred at room temperature for 15h, added water (300mL) to quench the reaction, extracted three times with dichloromethane (200mL), combined organic phases were washed with water (200mL), washed with saturated brine (200mL), separated, and the organic phase A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com