Feilike mixture extract and use thereof and quality control method
A technology for mixture and extract, applied in the field of quality control of Feilike mixture extract, can solve the problems of low transfer rate of baicalin, inconsistent preparation methods, no quality identification standards, etc., and achieves improvement of baicalin content and extraction method. Simple, control the effect of product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1, the preparation method of Feilike mixture extract
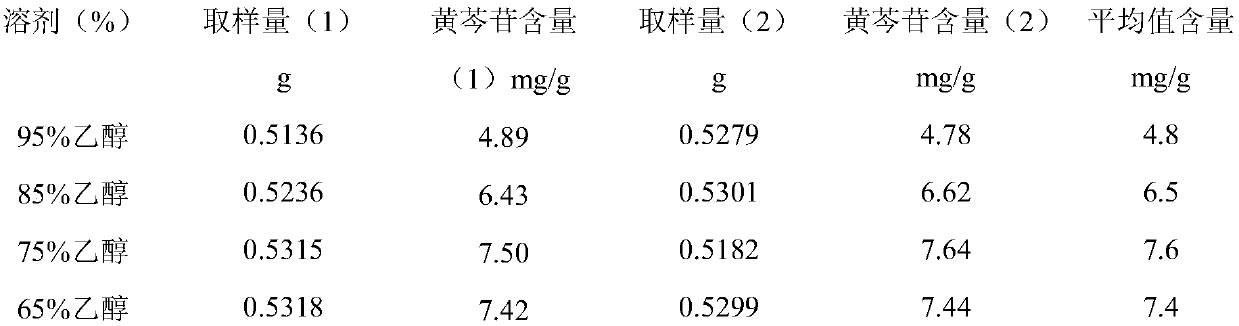
[0026] The preparation method of Feilike mixture extract comprises the steps of: taking 29g of Scutellaria baicalensis, 28g of Radix Peucedanum, 28g of Baibu, 28g of Gentiana safflower, 26g of Wutong root, 25g of Hedyotis diffusa, 23g of red pipe medicine according to the prescription, adding water Decoct twice, add boiling water equivalent to 7 times the weight of the medicinal material for 2 hours for the first time, add yu boiling water equivalent to 4 times the weight of the medicinal material for 1.5 hours, combine the decoction liquid, filter, and concentrate the filtrate to Clear paste with a relative density of 1.03 to 1.05 (80°C) is available. The clear paste obtained was brown. Every 1g of Scutellaria baicalensis contains baicalin (C 21 h 18 o 11 ), higher than 3.5mg. Adding boiling water directly in the preparation process helps to improve the conversion rate of baicalin.
[0027] Control...
Embodiment 2
[0028] Embodiment 2, Feilike mixture extract quality control method
[0029] (1) Identification of Radix Peucedanum: Take 3 parts of Feilike mixture extract prepared in Example 1, add water and dilute to 30ml, extract 2 times with ether, 25ml each time, combine the ether solution, evaporate to dryness, add ethyl acetate to the residue 1ml to dissolve, as the test solution.
[0030] Separately take 1 g of Peucedanum as reference medicinal material (batch number: 120951-200704, source: China Institute for the Control of Pharmaceutical and Biological Products), add 20 ml of ether, heat and reflux for 30 minutes, filter, evaporate the filtrate to dryness, add 1 ml of ethyl acetate to dissolve the residue, and use it as a control Medicinal solution.
[0031] And the extract of Herba Peucedanum was treated as the negative control solution according to the test sample.
[0032]According to the test of thin-layer chromatography (Appendix VI B of "Chinese Pharmacopoeia" 2010 Edition)...
Embodiment 3
[0047] Embodiment 3, detection method reliability
[0048] (1) Specificity
[0049] Preparation of negative solution: Get 0.5 g of the extractum lacking Scutellaria baicalensis medicinal material and put it in a 25ml volumetric flask, add an appropriate amount of 75% ethanol, and process according to the test solution to obtain a negative solution.
[0050] Determination method: according to the above-mentioned chromatographic conditions, respectively accurately absorb 10 μ l of the reference substance solution, the test solution, and the negative solution, and inject them into the liquid chromatograph. Requirements, the number of theoretical plates is greater than 2000 in terms of baicalin, the negative sample solution has no interference, and the specificity is strong.
[0051] (2) Linear relationship
[0052] Use the baicalin reference substance stock solution (0.3324mg / ml) to accurately draw (1ml, 2.5ml, 5ml, 8ml, 20ml), put them in 25ml volumetric flasks, add methanol t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com