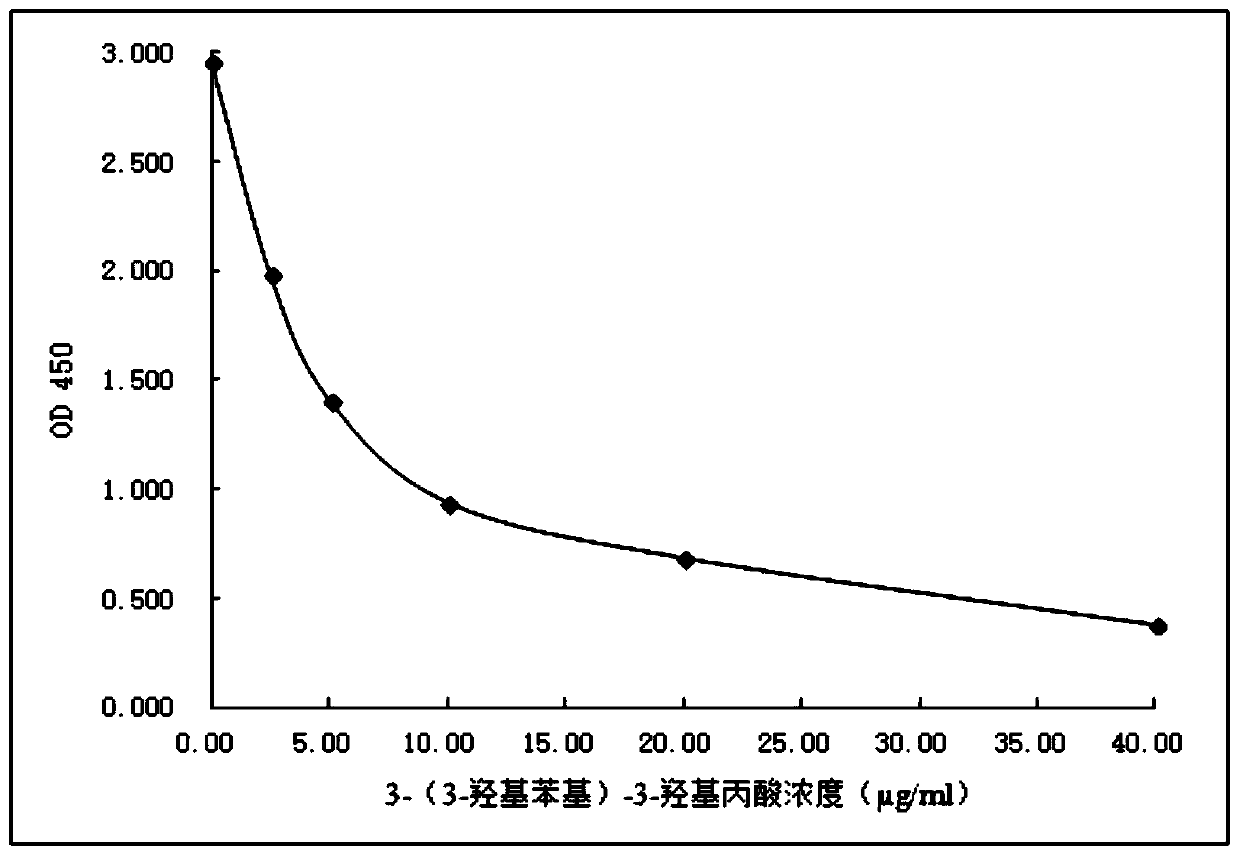
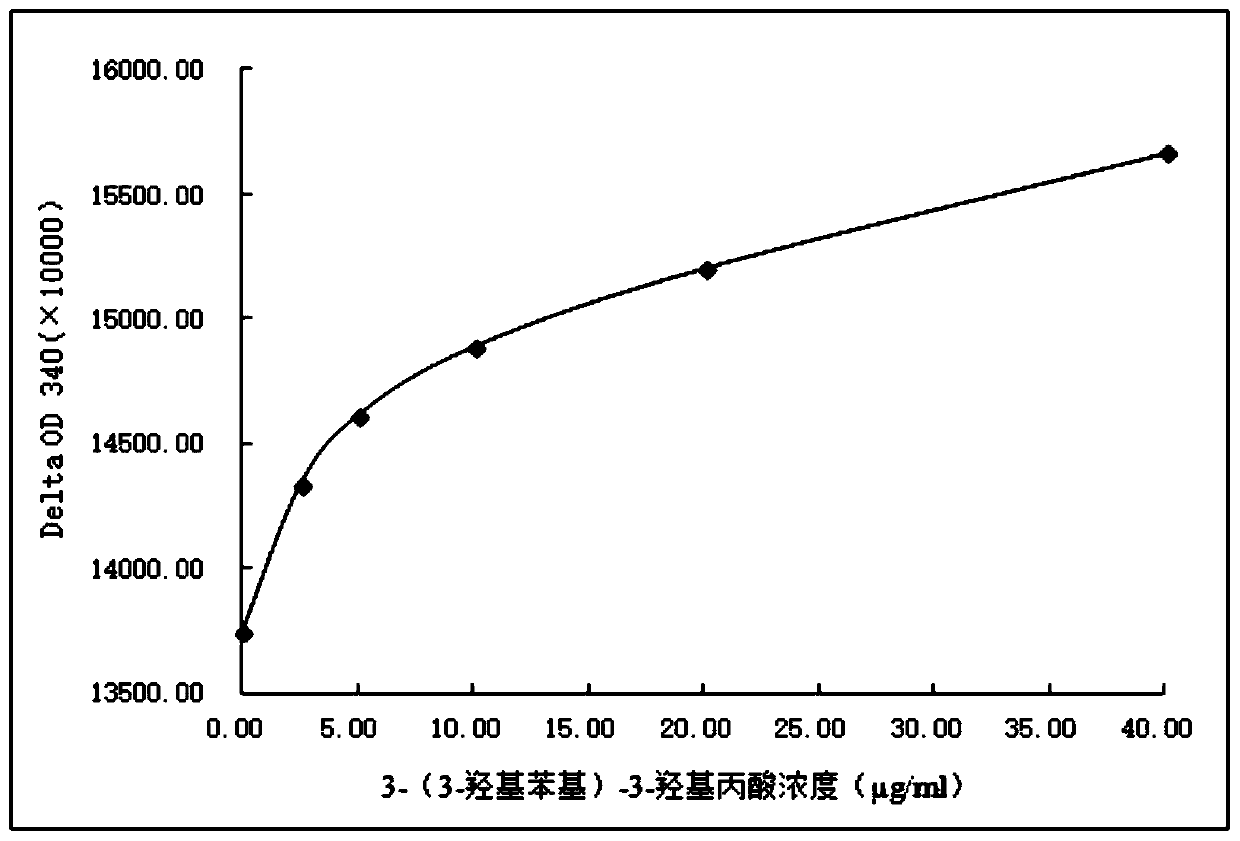
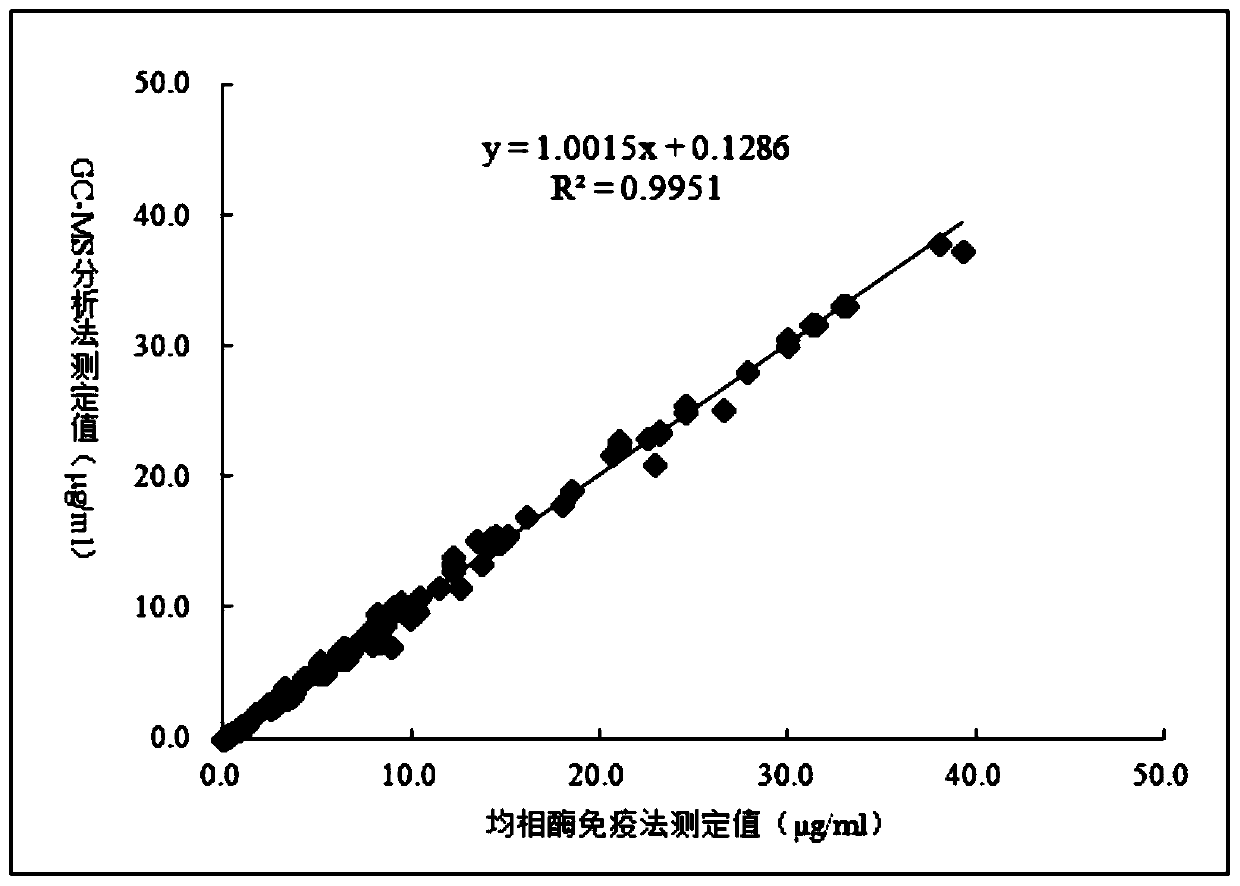
Derivative of 3-(3-hydroxylphenyl)-3-hydroxy-propionic acid, homogeneous enzyme immunodetection reagent and preparation method therefor
A technology of hydroxypropionase and homogeneous enzyme immunization, which can be used in the preparation of carboxylate, the preparation of organic compounds, the preparation of carboxylate/lactone, etc., and can solve the problems of complicated operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1. Synthesis of 3-(3-hydroxyphenyl)-3-hydroxypropionic acid derivative
[0079] The synthetic route of 3-(3-hydroxyphenyl)-3-hydroxypropionic acid derivative is as follows:
[0080]
[0081] The preparation process of 3-(3-hydroxyphenyl)-3-hydroxypropionic acid derivative is as follows:
[0082] (1) Synthesis of compound 3:
[0083] Weigh 20g of compound 1, namely 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (purchased from Sigma) and 33.2g of compound 2 (purchased from Sigma), and dissolve them in 300mL DMF together. Add 36.4g of K 2 CO 3 , The reaction mixture solution was prepared, and the above reaction mixture solution was stirred at room temperature for 12 hours. After the reaction was completed, 200 mL of purified water was added to the above reaction solution, and the solution was extracted with 300 mL of DCM. The extraction step was repeated 3 times. The organic phase obtained by extraction is passed through Na 2 SO 4 After drying, the residue obtained after co...
Embodiment 2
[0086] Example 2. Synthesis of 3-(3-hydroxyphenyl)-3-hydroxypropionic acid immunogen
[0087] The 3-(3-hydroxyphenyl)-3-hydroxypropionic acid immunogen in this example is formed by linking the 3-(3-hydroxyphenyl)-3-hydroxypropionic acid derivative obtained in Example 1 with BSA, Its structural formula is shown in the following formula (II):
[0088]
[0089] The carrier is BSA.
[0090] The specific steps of the synthesis method of the 3-(3-hydroxyphenyl)-3-hydroxypropionic acid immunogen are as follows:
[0091] (1) Weigh 2.72g potassium dihydrogen phosphate, 4.26g disodium hydrogen phosphate, 8.5g sodium chloride, 0.95g magnesium chloride, and dissolve them in 1L of deionized water, adjust the pH to 8.2, and prepare buffer A;
[0092] (2) Weigh 3 mg of BSA and dissolve it in 3 mL of the above buffer A at -4°C to make a 1 mg / mL carrier solution;
[0093] (3) Weigh 3 mg of the 3-(3-hydroxyphenyl)-3-hydroxypropionic acid derivative obtained in Example 1, and dissolve it in 300 μl of the ...
Embodiment 3
[0096] Example 3. Synthesis of 3-(3-hydroxyphenyl)-3-hydroxypropionic acid immunogen
[0097] The 3-(3-hydroxyphenyl)-3-hydroxypropionic acid immunogen in this example is obtained by linking the 3-(3-hydroxyphenyl)-3-hydroxypropionic acid derivative obtained in Example 1 with ovalbumin. The structural formula is as shown in the following formula (Ⅱ)
[0098]
[0099] The carrier is ovalbumin.
[0100] The specific steps of the synthesis method of the 3-(3-hydroxyphenyl)-3-hydroxypropionic acid immunogen are as follows:
[0101] (1) Weigh 2.3g of potassium dihydrogen phosphate, 3.8g of disodium hydrogen phosphate, 8.0g of sodium chloride, and 0.7g of magnesium chloride, and dissolve them in 0.9L of deionized water, adjust the pH to 8.0, and prepare buffer A;
[0102] (2) Weigh 6 mg of ovalbumin and dissolve it in 3 mL of buffer A at -4°C to make a 2 mg / mL carrier solution;
[0103] (3) Weigh 2.4 mg of the 3-(3-hydroxyphenyl)-3-hydroxypropionic acid derivative obtained in Example 1, and d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com