Application of salvianolic acid A to preparation of hyperuricaemia and gout resisting drugs
A technology for hyperuricemia and uric acid, applied in the field of medicine, can solve unreported problems and achieve the effects of reducing serum uric acid levels, treatment prevention and treatment safety, and reducing mRNA expression levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
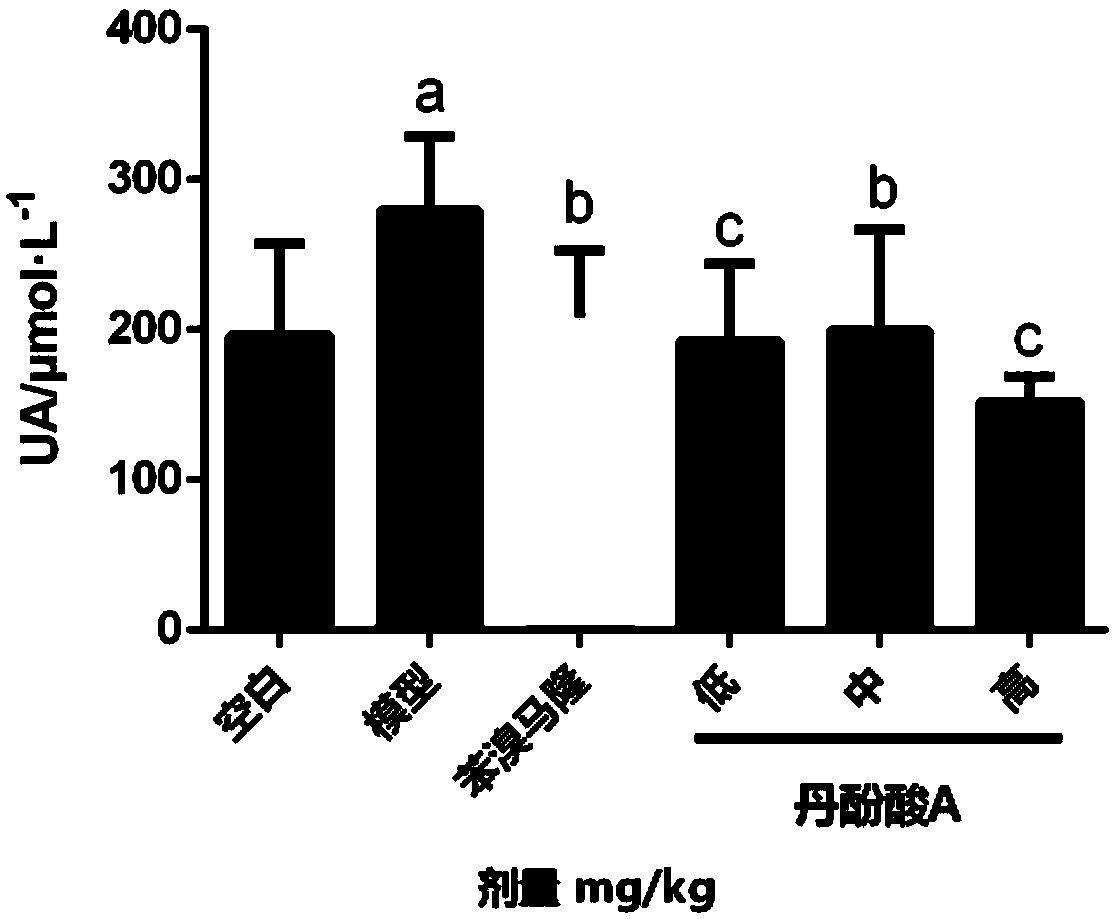
[0035] Experimental example 1. Salvianolic acid A reduces serum uric acid (UA) level in hyperuricemia model mice.
[0036]Experimental materials: Kunming mice were purchased from Beijing Huafukang Biotechnology Co., Ltd. Potassium oxonate, salvianolic acid A, allopurinol, and benzbromarone were purchased from Sigma-Aldrich, Germany. Sodium carboxymethyl cellulose was purchased from Sinopharm Chemical Reagent Co., Ltd. The uric acid kit was purchased from Zhongsheng Beikong Biotechnology Co., Ltd.
[0037] Solution preparation: 1% sodium carboxymethylcellulose was dissolved, boiled, cooled and used as a solvent to dissolve salvianolic acid A, potassium oxonate, allopurinol and benzbromarone respectively to form a suspension.
[0038] Experimental grouping: 18-20g mice were randomly divided into normal control group, model group, benzbromarone group (25mg·kg -1 , positive control), salvianolic acid A low, medium and high dose groups (3, 10, 30 mg·kg -1 ), 10 in each group. ...
Embodiment 2
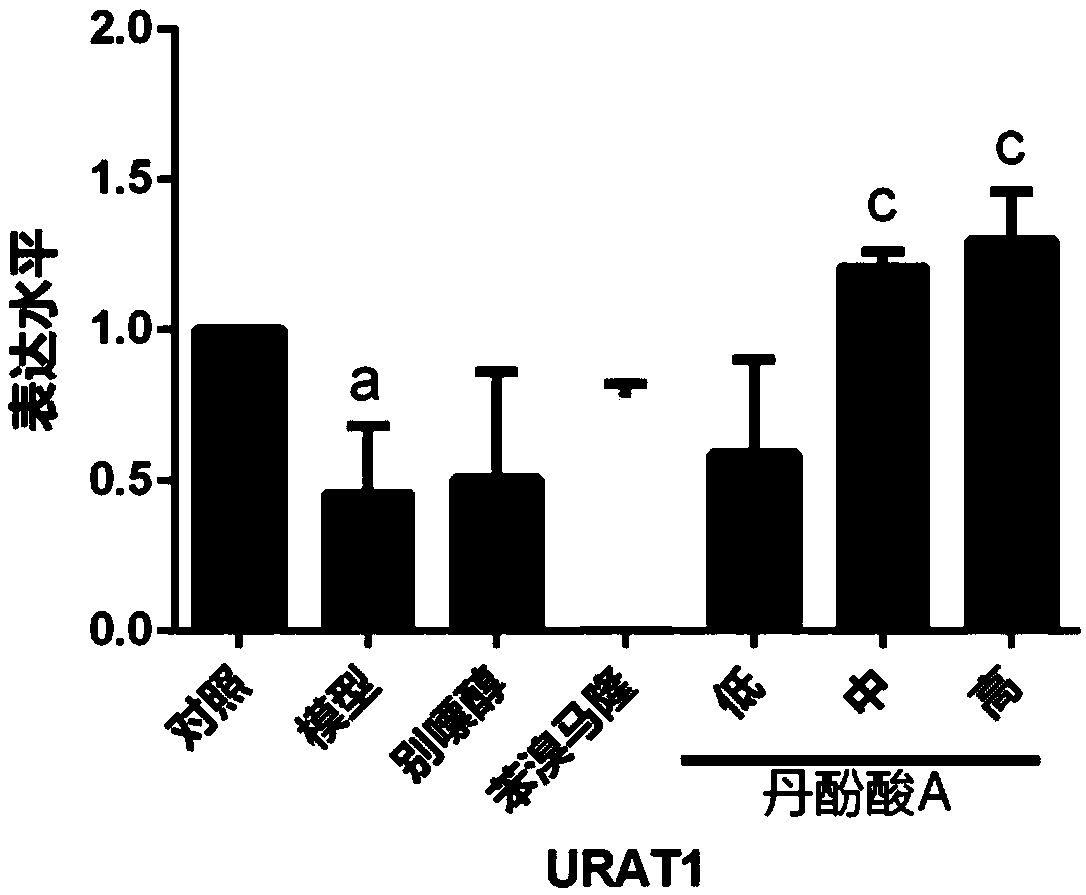
[0044] Example 2. Effect of salvianolic acid A on the mRNA expression level of urate anion transporter 1 (URAT1) in hyperuricemia model mice. n=3.
[0045] Experimental grouping: 18-20g mice were randomly divided into normal control group, model group, allopurinol group (25mg·kg -1 , positive control), benzbromarone group (25mg·kg -1 , positive control), salvianolic acid A low, medium and high dose groups (3, 10, 30 mg·kg -1 ), 10 in each group. The relevant solution preparation and experimental scheme are the same as in Example 1. The reverse transcription kit and SYBR green dye were purchased from Biotech Co., Ltd. (TAKARA, Japan). Primers were purchased from Sangon Bioengineering Co., Ltd., and the purification method was HAP.
[0046] Table 2. URAT1 primers and internal reference primer sequences.
[0047]
[0048] After the mice were sacrificed, the kidneys of the mice were taken, and the total RNA in the kidneys was extracted using the Trizol method, and the RN...
Embodiment 3
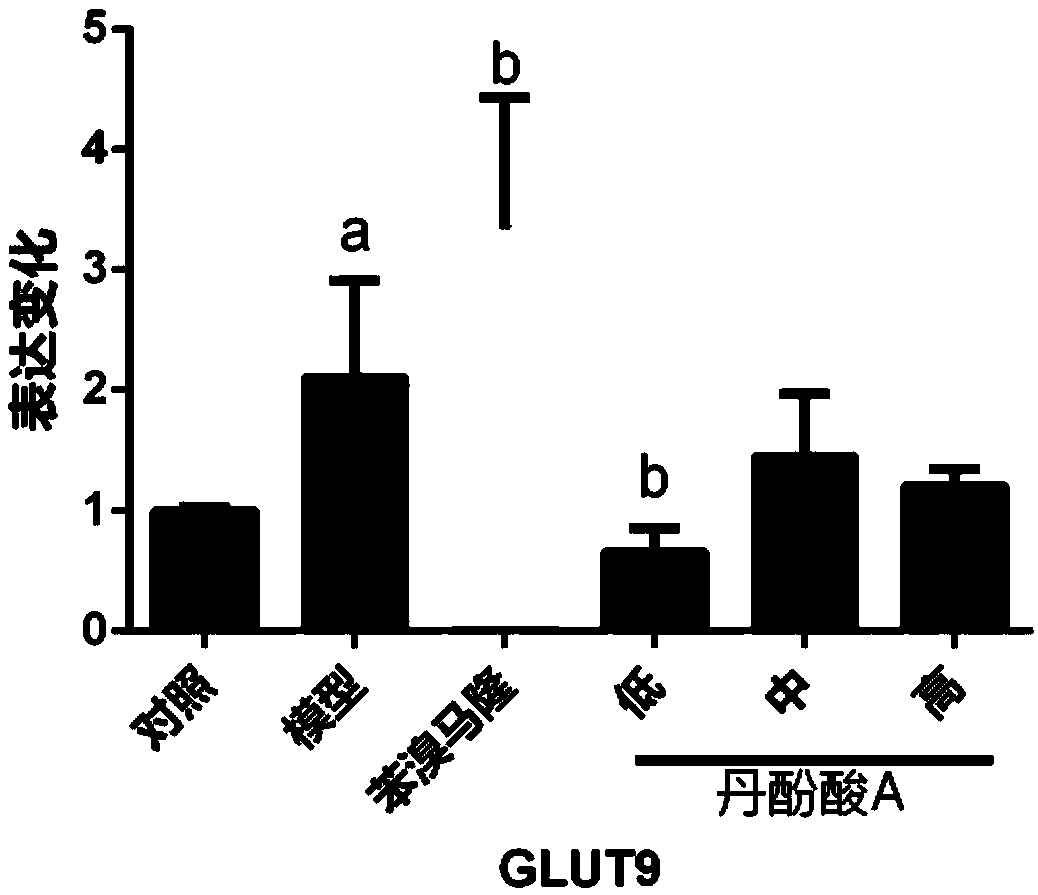
[0053] Example 3. Effect of salvianolic acid A on the mRNA expression level of glucose transporter 9 (GLUT9) in hyperuricemia model mice. ( n=3).
[0054] Experimental grouping: 18-20g mice were randomly divided into normal control group, model group, benzbromarone group (25mg·kg -1 , positive control), salvianolic acid A low, medium and high dose groups (3, 10, 30 mg·kg -1 ), 10 in each group. The relevant solution preparation and experimental scheme are the same as in Example 2. The reverse transcription kit and SYBR green dye were purchased from Biotech Co., Ltd. (TAKARA, Japan). Primers were purchased from Sangon Bioengineering Co., Ltd., and the purification method was HAP.
[0055] Table 4. GLUT9 primers and internal reference primer sequences.
[0056]
[0057] After the mice were sacrificed, the kidneys of the mice were taken, and the total RNA in the kidneys was extracted using the Trizol method, and the RNA was quantified with a UV spectrophotometer. SYBRG...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com