Terpyridine derivative and preparation method thereof, and applications of terpyridine derivative in organic electroluminescent devices
A technology of terpyridine and its derivatives, which is applied in the preparation method and application, can solve the problems such as difficult to precisely control the doping and increase the complexity of the device, and achieve the effects of low driving voltage, low efficiency roll-off, and high fluorescence quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
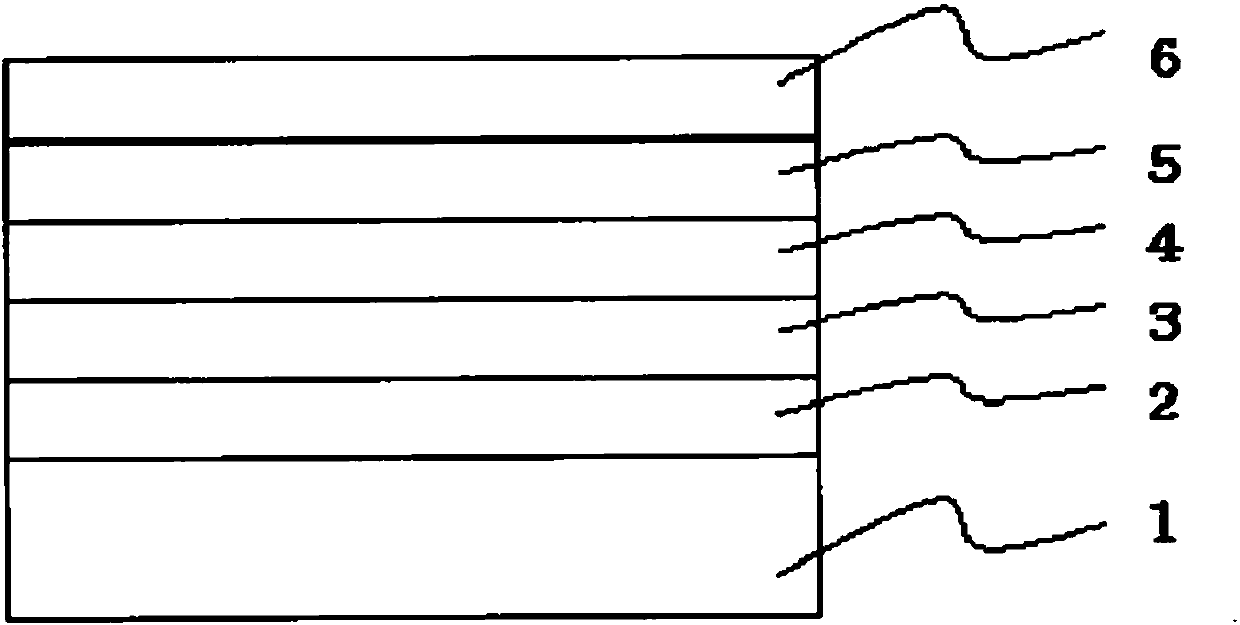
Image
Examples
Embodiment 1
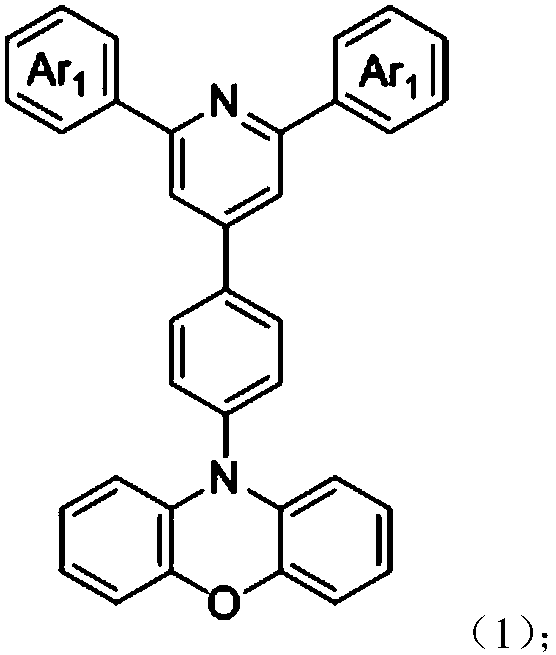
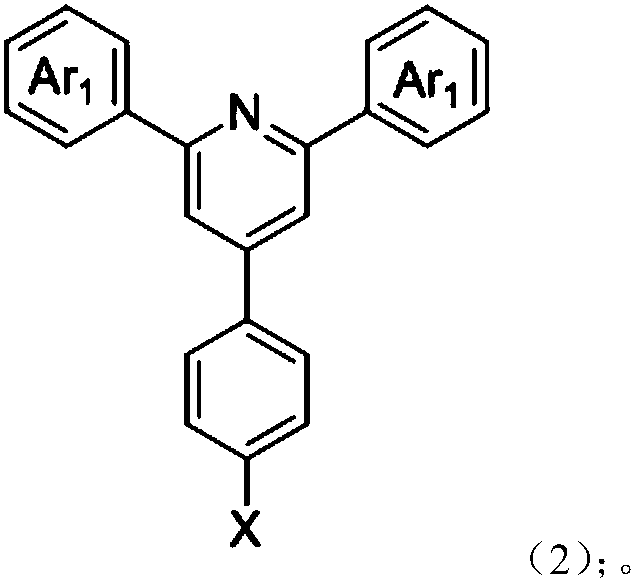
[0031] Synthesis of Example 1: Under argon protection, 0.78 g of 4-bromophenyl terpyridine derivatives (intermediate 1), 0.38 g of phenoxazine, and 15 mg of palladium acetate were added to a 100 mL two-port reactor equipped with a reflux tube. 0.5 mL of a toluene solution of 10% tri-tert-butylphosphine was added, and 60 mL of toluene was added. The resulting solution was heated to 90°C and stirred for 12 hours. After cooling to room temperature, the organic solvent was distilled off. The organic phase was extracted by adding a large amount of water and dichloromethane and dried over anhydrous sodium sulfate. After distilling off the organic liquid phase, the product was purified by column chromatography with dichloromethane and methanol. After drying, 800 mg of yellow-green powder was obtained, with a yield of 81.5%.
[0032] Product characterization: 1 H NMR (400MHz, DMSO-d 6 )δ9.60–9.59(m,2H),8.80–8.75(m,2H),8.75–8.72(m,2H),8.52(s,2H),8.45(d,J=8.5Hz,2H),7.67 (d,J=8.5Hz,...
Embodiment 2
[0036] Synthesis of Example 2: Under argon protection, 0.78 g of 4-bromophenyl terpyridine derivatives (intermediate 2), 0.38 g of phenoxazine, and 15 mg of palladium acetate were added to a 100 mL two-port reactor equipped with a reflux tube. 0.5 mL of a toluene solution of 10% tri-tert-butylphosphine was added, and 60 mL of toluene was added. The resulting solution was heated to 90°C and stirred for 12 hours. After cooling to room temperature, the organic solvent was distilled off. The organic phase was extracted by adding a large amount of water and dichloromethane and dried over anhydrous sodium sulfate. After distilling off the organic liquid phase, the product was purified by column chromatography with dichloromethane and methanol. After drying, 820 mg of yellow powder was obtained, with a yield of 83.5%.
[0037] Product Characterization: 1 H NMR (600MHz, DMSO-d 6)δ9.75(s,4H),9.30(s,2H),8.63(s,2H),8.42(d,J=8.5Hz,2H),7.65(d,J=8.4Hz,2H),6.76– 6.74(m,2H),6.69–6.66(m,4...
Embodiment 3
[0041] Synthesis of Example 3: Under argon protection, 0.78 g of 4-bromophenyl terpyridine derivative (intermediate 3), 0.38 g of phenoxazine, and 15 mg of palladium acetate were added to a 100 mL two-port reactor equipped with a reflux tube, 0.5 mL of a toluene solution of 10% tri-tert-butylphosphine was added, and 60 mL of toluene was added. The resulting solution was heated to 90°C and stirred for 12 hours. After cooling to room temperature, the organic solvent was distilled off. The organic phase was extracted by adding a large amount of water and dichloromethane and dried over anhydrous sodium sulfate. After distilling off the organic liquid phase, the product was purified by column chromatography with dichloromethane and methanol. After drying, 900 mg of orange powder was obtained with a yield of 91.8%.
[0042] Product Characterization: 1 H NMR (600MHz, Chloroform-d) δ10.00(s, 2H), 9.42(d, J=5.3Hz, 2H), 8.26(dd, J=5.4, 2.3Hz, 2H), 8.21(s, 2H) ,7.98(d,J=7.9Hz,2H),7.6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Light emitting area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com