Orange-red fluorescent zinc coordination polymer with mixed ligands, preparation method of polymer and application of polymer
A technology of zinc coordination polymers and mixed ligands, applied in zinc organic compounds, fluorescence/phosphorescence, luminescent materials, etc., to achieve the effects of simple and feasible preparation conditions, strong practicability, and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 Preparation of coordination polymer of the present invention
[0034] Take material according to the following specific mass or volume: Htpc (8.3mg, 0.03mmol), H 3 ntb (11.8mg, 0.03mmol), Zn (NO 3 ) 2 ·6H 2 O (17.8 mg, 0.06 mmol), CH 3 CN (2mL), H 2 O (8 mL). Put the above materials in a 25mL reaction kettle, stir for 0.5-1.5h, put the reaction system in a constant temperature oven at 140°C, react for 6 days, and cool it to room temperature naturally, and observe orange-red rod-shaped crystals, filter them out from the mother liquor, Wash with distilled water and dry naturally ( figure 2 ).
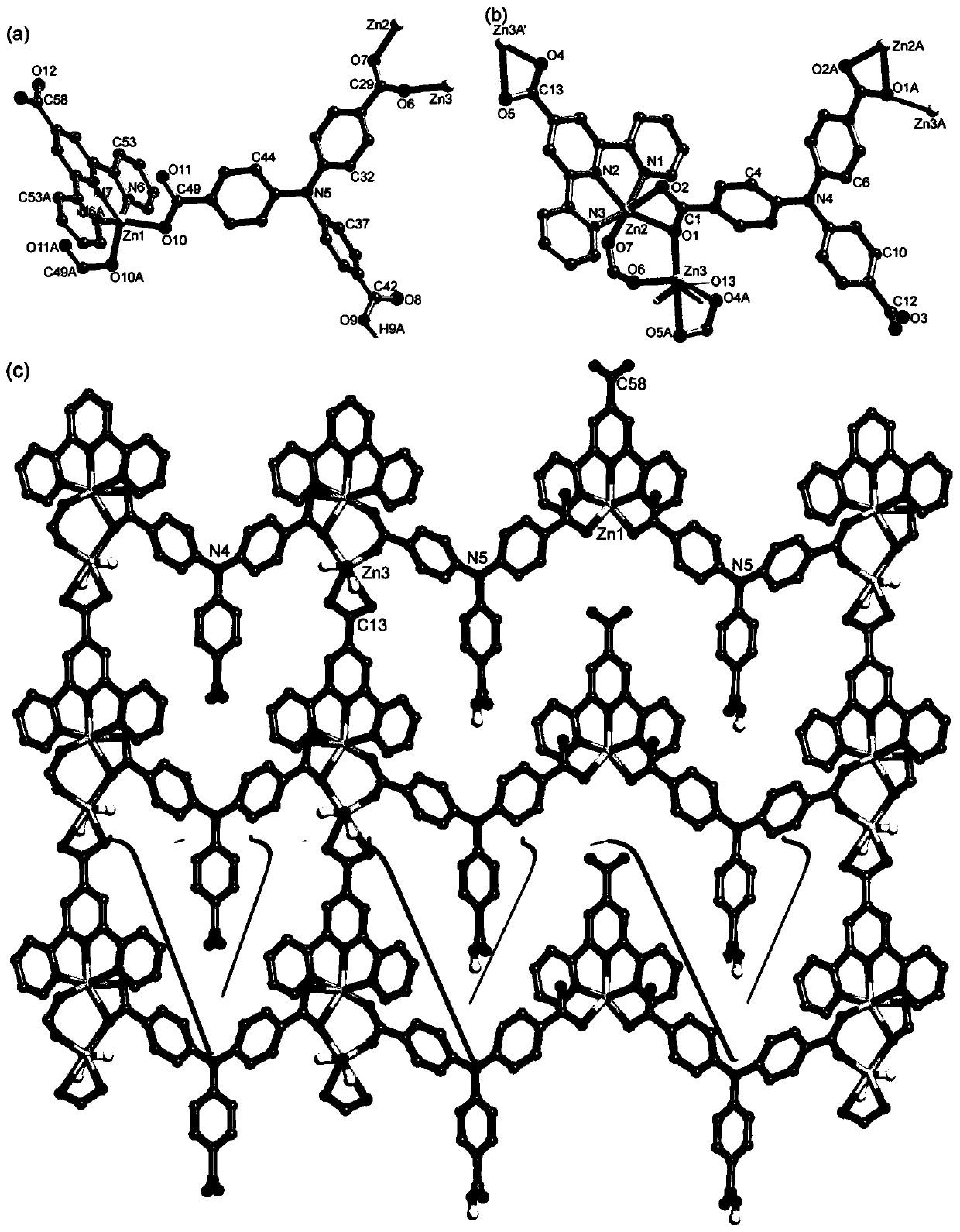
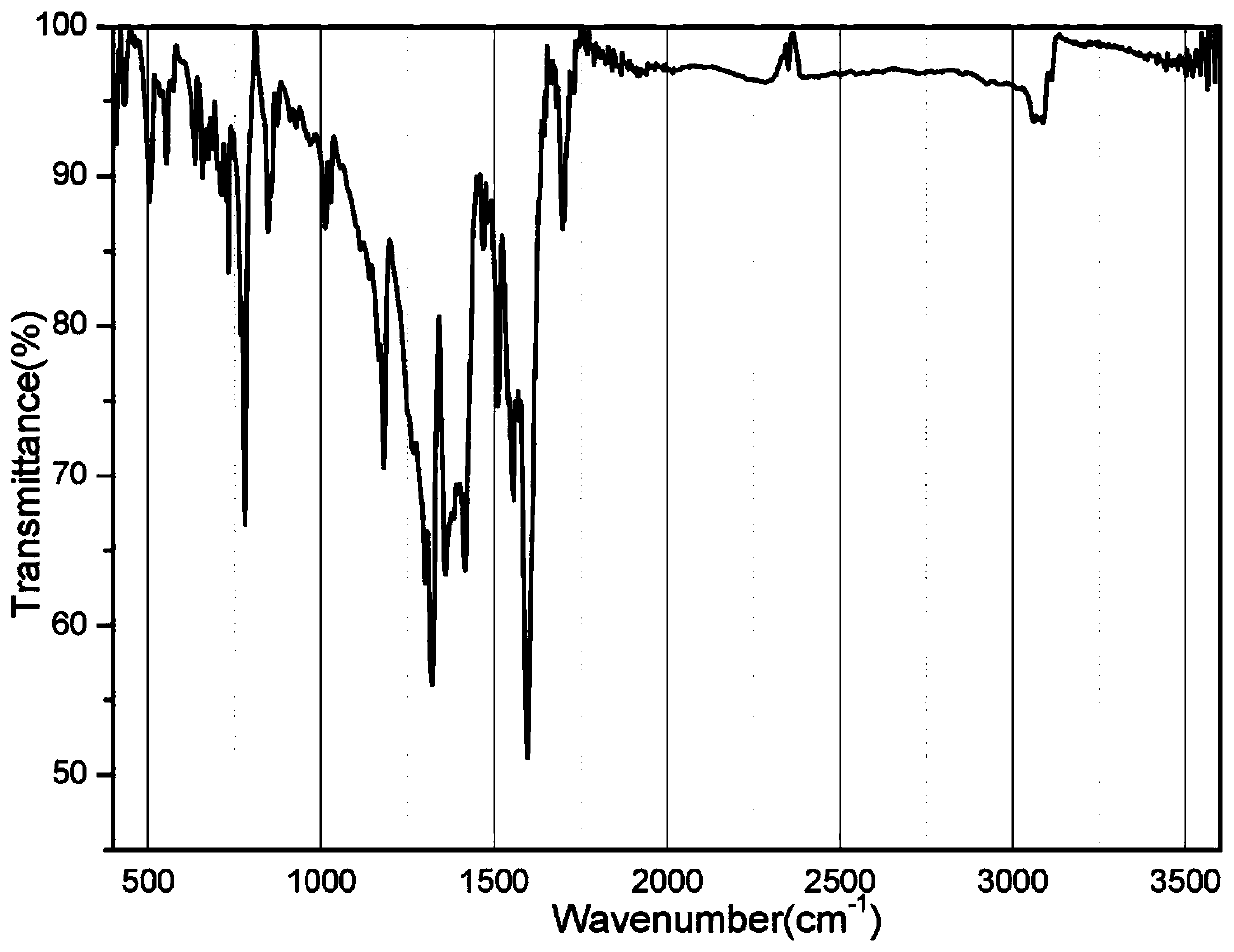
[0035] The infrared spectrum of the crystal sample was taken by Nicolet Impact 410FTIR spectrometer with KBr as the base at 400-4000cm -1 range (see image 3 ). Carry out powder diffraction test with Shimadzu XRD-6100 type X-ray diffractometer, the peak of test collection of spectra matches the peak energy of crystal structure simulation collection of collec...
Embodiment 2
[0045] The preparation of embodiment 2 complexes of the present invention
[0046] Get material according to the following specific quality or volume: Htpc (41.6mg, 0.15mmol), H 3 ntb (11.8mg, 0.03mmol), Zn (NO 3 ) 2 ·6H 2 O (26.8 mg, 0.09 mmol), CH 3 CN (3mL), H 2 O (7 mL).
[0047] The above materials were placed in a 25mL reactor, stirred for 0.5-1.5h, reacted in a constant temperature oven at 150°C for 4 days, and cooled to room temperature naturally to obtain crystals, which were filtered out from the mother liquor, washed with distilled water, and dried naturally.
[0048] The X-ray powder diffraction data comparison of product (see Figure 4 ), the obtained data is similar to that of Example 1, illustrating that the crystal structure made by Example 2 does not change and the product has high purity.
[0049] This embodiment is repeated many times, obtain [Zn according to actual production 5 (tpc) 3 (ntb)(Hntb) 2 (H 2 O) 2 ] n The mass of 10.6mg ~ 14.7mg, ba...
Embodiment 3
[0050] The preparation of embodiment 3 complexes of the present invention
[0051] Take material according to the following specific mass or volume: Htpc (8.3mg, 0.03mmol), H 3 ntb (11.8mg, 0.03mmol), Zn (NO 3 ) 2 ·6H 2 O (44.6 mg, 0.15 mmol), CH 3 CN (5mL), H 2 O (5 mL).
[0052] The above materials were placed in a 25mL reactor, reacted in a constant temperature oven at 160°C for 7 days, and then cooled to room temperature naturally to obtain red crystals, which were filtered out from the mother liquor, washed with distilled water, and dried naturally.
[0053] The X-ray powder diffraction data comparison of product (see Figure 4 ), the obtained data is similar to that of Example 1, indicating that the crystal structure obtained in Example 3 does not change and the product has high purity.
[0054] This embodiment is repeated many times, obtain [Zn according to actual production 5 (tpc) 3 (ntb)(Hntb) 2 (H 2 O) 2 ] n The mass of 8.5mg ~ 11.8mg, based on H 3 The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com