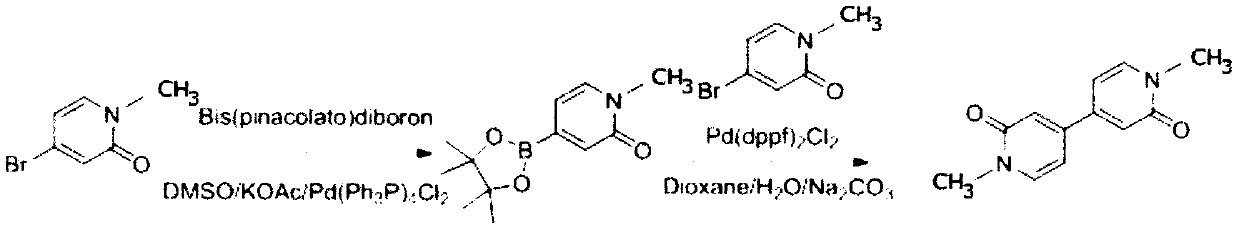
Preparation method of 1,1'-dimethyl-2,2'-diketone-4,4'-dipyridyl
A kind of methyl pyridine and dimethyl technology, applied in the field of organic synthesis, can solve the problem that the synthesis method cannot be universal, and achieve the effects of improving the utilization rate of workshop equipment, simple reaction system and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A preparation method of 1,1'-dimethyl-2,2'-diketone-4,4'-bipyridine, comprising the following steps:
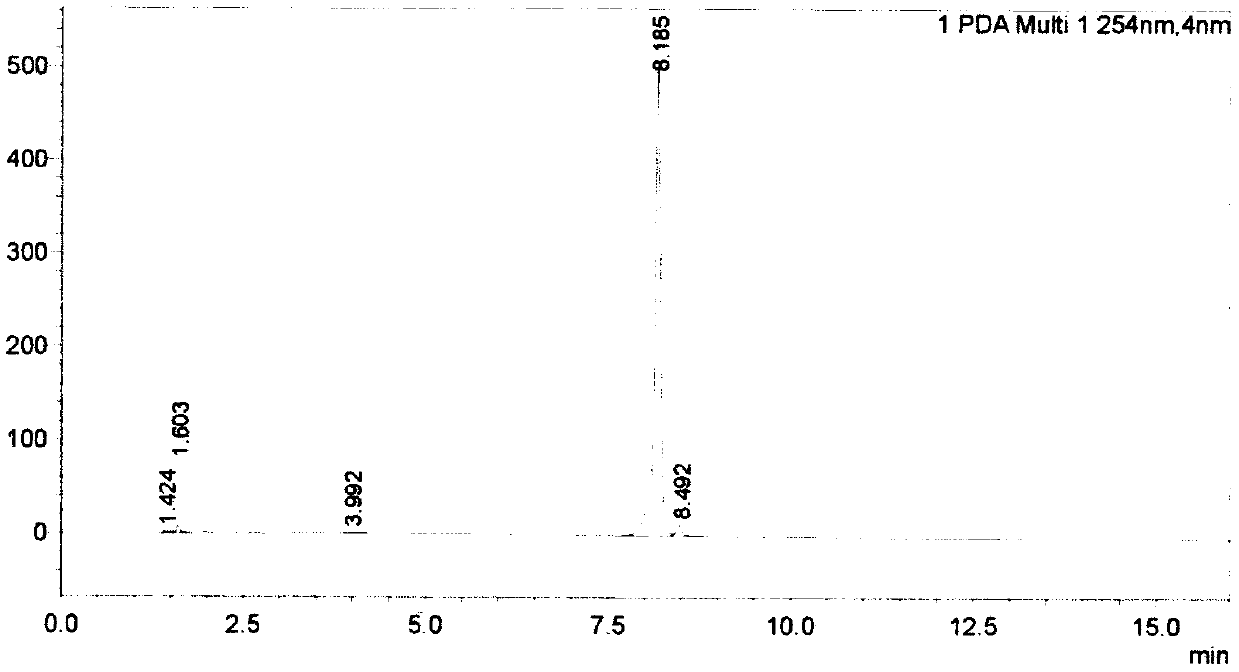
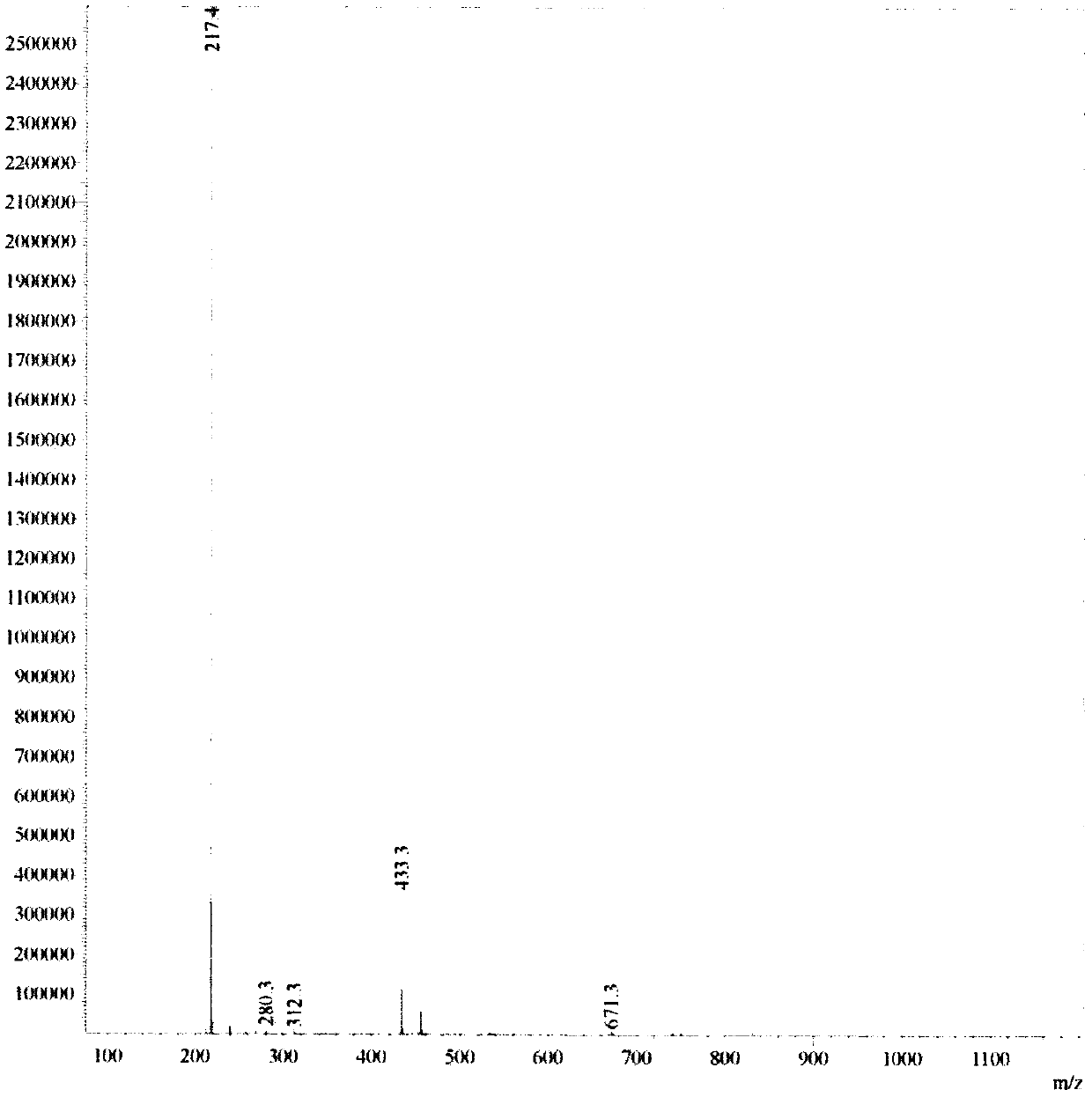
[0027] (1) Dissolve 18.6g of 4-bromo-1-methylpyridin-2-one in 250 mL of DMSO, stir well, then add 14.8g of potassium acetate, 30.5g of pinacol diborate and 7.0g of Pd( dppf) 2 Cl 2 , under the protection of nitrogen, reflux at 80°C overnight, TLC point plate to detect the reaction end point, then the reaction solution flows through the silica gel column for separation, and the column is rinsed with a mixed solution of methanol and dichloromethane with a volume ratio of 1:10 to collect the target The product was evaporated to dryness with a rotary evaporator to remove the organic solvent to obtain 9.0 g of compound I.
[0028] (2) 7.05g of compound I obtained in step (1) and 6.2g of 4-bromo-1-methylpyridin-2-one, 6.3g of sodium carbonate and 2.4g of tetrakistriphenylphosphine palladium dichloride were successively dissolved in 200mL Dioxane and 40mL water were stirre...
Embodiment 2
[0032] A preparation method of 1,1'-dimethyl-2,2'-diketone-4,4'-bipyridine, comprising the following steps:
[0033] (1) Dissolve 18.2g of 4-bromo-1-methylpyridin-2-one in 260 mL of DMSO, stir well, then add 14.1g of potassium acetate, 30.2g of pinacol diborate and 7.2g of Pd( dppf) 2 Cl 2 , under the protection of nitrogen, reflux at 90°C overnight, TLC point plate to detect the reaction end point, then the reaction solution flows through the silica gel column for separation, and the column is rinsed with a mixed solution of methanol and dichloromethane with a volume ratio of 1:5 to collect the target The product was evaporated to dryness with a rotary evaporator to remove the organic solvent to obtain 9.1 g of compound I.
[0034] (2) 7.5g of compound I obtained in step (1) and 6.5g of 4-bromo-1-methylpyridin-2-one, 6.2g of sodium carbonate and 2.5g of tetrakistriphenylphosphine palladium dichloride were dissolved in 210mL successively Dioxane and 50mL water were stirred ...
Embodiment 3
[0038] A preparation method of 1,1'-dimethyl-2,2'-diketone-4,4'-bipyridine, comprising the following steps:
[0039] (1) Dissolve 17.8g of 4-bromo-1-methylpyridin-2-one in 240 mL of DMSO, stir well, then add 13.9g of potassium acetate, 30.1g of biboronic acid pinacol ester and 7.8g of Pd( dppf) 2 Cl 2 , under the protection of nitrogen, reflux at 100°C overnight, TLC point plate to detect the reaction end point, then the reaction solution flows through the silica gel column for separation, and the column is rinsed with a mixed solution of methanol and dichloromethane with a volume ratio of 2:5 to collect the target The product was evaporated to dryness with a rotary evaporator to remove the organic solvent to obtain 9.3 g of compound I.
[0040] (2) 7.8g of compound I obtained in step (1) and 6.6g of 4-bromo-1-methylpyridin-2-one, 6.8g of sodium carbonate and 2.7g of tetrakistriphenylphosphine palladium dichloride were successively dissolved in 200mL Dioxane and 50mL water ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com