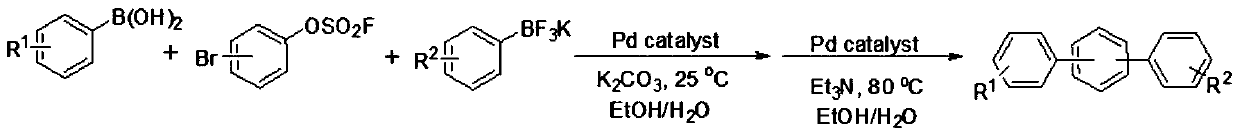
A kind of method utilizing bromoarylsulfonyl fluoride to prepare unsymmetrical terphenyl compound
A sulfonyl fluoride, asymmetric technology, applied in the field of preparing asymmetric terphenyl compounds, can solve the problems of complicated operation, low product yield, harsh reaction conditions, etc., to simplify the operation process, simplify the reaction operation steps, increase the Effects of synthetic pathways
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
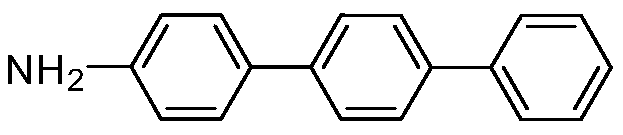
[0019] Embodiment 1: the preparation of 4-aminoterphenyl
[0020]
[0021] In air, add 1mmol 4-bromoarylsulfonyl fluoride, 1mmol 4-aminophenylboronic acid, 1mmol potassium phenyltrifluoroborate, 0.01mmol palladium acetate, 2mmol potassium carbonate, 4mL ethanol, 4mL water into a round bottom flask In the reaction, stir at 25°C for 1 hour, then add 2mmol triethylamine, 0.01mmol palladium acetate to the reaction, and increase the temperature to 80°C, react for 2 hours, after the reaction, add ethyl acetate to quench the reaction, the reaction The mixture was concentrated by a rotary evaporator and separated by column chromatography to obtain an analytically pure asymmetric terphenyl compound with a yield of 88%. The structure of the product was confirmed by H NMR and C NMR analysis. 1H NMR (400MHz, CDCl 3 )δ7.63(d, J=7.6Hz, 6H), 7.51–7.38(m, 5H), 7.34(t, J=7.3Hz, 1H), 6.77(d, J=8.3Hz, 2H), 4.05( s,2H). 13 C NMR (101MHz, CDCl 3 )δ152.0, 145.2, 144.8, 143.0, 133.6, 132.2, 1...
Embodiment 2
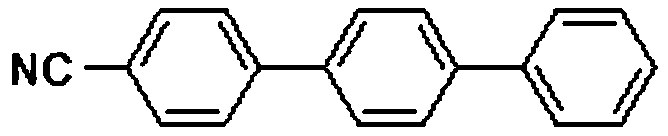
[0022] Embodiment 2: the preparation of 4-cyano p-terphenyl
[0023]
[0024] In air, add 1mmol 4-bromoarylsulfonyl fluoride, 1mmol 4-cyanophenylboronic acid, 1mmol potassium phenyltrifluoroborate, 0.01mmol palladium acetate, 2mmol potassium carbonate, 4mL ethanol, 4mL water into the round bottom In the flask, the reaction was carried out with stirring at 25° C. for 1 hour, then 3 mmol triethylamine and 0.01 mmol palladium acetate were added to the reaction, and the temperature was increased to 80° C., and the reaction was carried out for 2 hours. After the reaction was completed, ethyl acetate was added to quench the reaction. The reaction mixture was concentrated with a rotary evaporator and separated by column chromatography to obtain an analytically pure asymmetric terphenyl compound with a yield of 92%. The structure of the product was confirmed by H NMR and C NMR analysis. 1H NMR (400MHz, CDCl 3 )δ7.79–7.60(m,10H),7.48(t,J=7.5Hz,2H),7.39(t,J=7.4Hz,1H). 13 C NMR (101...
Embodiment 3
[0025] Embodiment 3: the preparation of 4-formyl p-terphenyl
[0026]
[0027] In air, add 1mmol 4-bromoarylsulfonyl fluoride, 1mmol 4-formylphenylboronic acid, 1mmol potassium phenyl trifluoroborate, 0.01mmol palladium acetate, 4mmol potassium carbonate, 4mL ethanol, 4mL water into the round bottom In the flask, the reaction was carried out with stirring at 25° C. for 1 hour, then 2 mmol triethylamine and 0.01 mmol palladium acetate were added to the reaction, and the temperature was increased to 80° C., and the reaction was carried out for 2 hours. After the reaction was completed, ethyl acetate was added to quench the reaction. The reaction mixture was concentrated by a rotary evaporator and separated by column chromatography to obtain an analytically pure asymmetric terphenyl compound with a yield of 81%. The structure of the product was confirmed by H NMR and C NMR analysis. 1H NMR (400MHz, CDCl 3 )δ10.07(s,1H),7.98(d,J=8.1Hz,2H),7.81(d,J=8.1Hz,2H),7.73(s,4H),7.69–7.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com