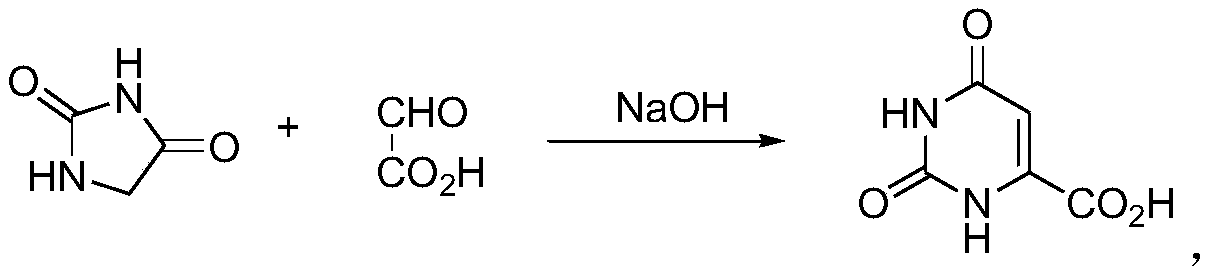
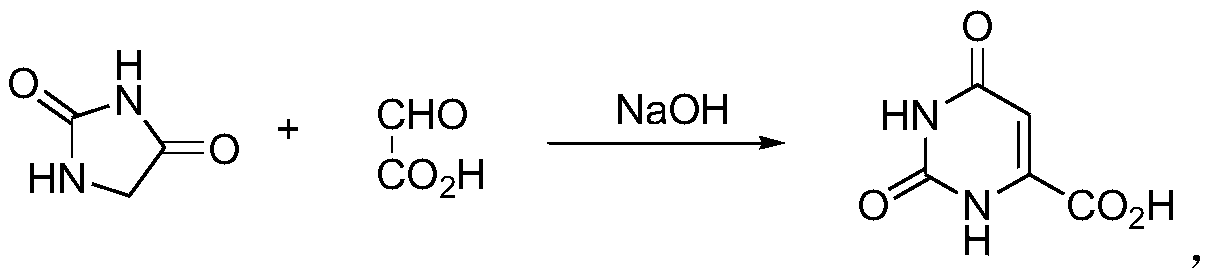
Improved synthesis method of orotic acid
A synthetic method, orotic acid technology, applied in the direction of organic chemistry, can solve the problems of non-compliance with environmental protection requirements, use of dangerous reagents, high cost of raw materials, etc., achieve continuous operation of specific surface area, increase of reaction yield, and reduce production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0033] A kind of improved synthetic method of orotic acid:
[0034] S1. Control the temperature in the reaction channel at 115-120°C, adjust the flow rate of the double pumps, and pump 1.5 kg of hydantoin, 10 kg of water, 2.5 kg of 50% glyoxylic acid mixed solution and 7.5 liters of 8N sodium hydroxide solution at the same time After 2 hours of heating cycle reaction, stop heating. When the temperature drops to 70-80°C, transfer the reaction solution to a 50-liter purification reactor, maintain the internal temperature at 60-65°C, and add concentrated hydrochloric acid dropwise to adjust the pH to 9 -9.5, centrifuge the precipitated solid to obtain a crude product of off-white sodium orotate, add the crude product to 6 liters of water, keep warm at 60-65°C, add concentrated hydrochloric acid to adjust the pH to 1-2, stir for half an hour, and The centrifugation of the solid that separates out obtains 3 kilograms of orotic acid crude product;
[0035] S2. Add the above crude s...
experiment example 2
[0037] A kind of improved synthetic method of orotic acid:
[0038] S1. Control the temperature in the reaction channel at 100-110°C, adjust the flow rate of the double pumps, and pump 1.5 kg hydantoin, 10 kg water, 2.5 kg 50% glyoxylic acid mixed solution and 7.5 liters of 8N sodium hydroxide at the same time Solution, after heating and circulating for 5 hours, stop heating, and when the temperature drops to 70-80°C, transfer the reaction solution to a 50-liter purification reactor, maintain the internal temperature at 50-60°C, and add concentrated hydrochloric acid dropwise to adjust the pH to 9-9.5, centrifuge the precipitated solid to obtain a crude product of off-white sodium orotate, add the crude product to 5 liters of water, keep it warm at 60-65°C, add concentrated hydrochloric acid dropwise to adjust the pH to 1-2, and stir for half an hour, The precipitated solid is centrifuged to obtain 2.8 kilograms of orotic acid crude product;
[0039]S2. Add the above crude so...
experiment example 3
[0041] A kind of improved synthetic method of orotic acid:
[0042] S1. Control the temperature in the reaction channel at 110-115°C, adjust the flow rate of the double pumps, and pump 1.5 kg of hydantoin, 10 kg of water, 2.5 kg of 50% glyoxylic acid mixed solution and 7.5 liters of 8N sodium hydroxide at the same time Solution, after heating and circulating for 2 hours, stop heating, and when the temperature drops to 70-80°C, transfer the reaction solution to a 50-liter purification reactor, maintain the internal temperature at 60-65°C, and add concentrated hydrochloric acid dropwise to adjust the pH to 9-9.5, centrifuge the precipitated solid to obtain a crude product of off-white sodium orotate, add the crude product to 5 liters of water, keep it warm at 60-65°C, add concentrated hydrochloric acid dropwise to adjust the pH to 1-2, and stir for half an hour, The precipitated solid is centrifuged to obtain 2.95 kilograms of orotic acid crude product;
[0043] S2. Add the abo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com