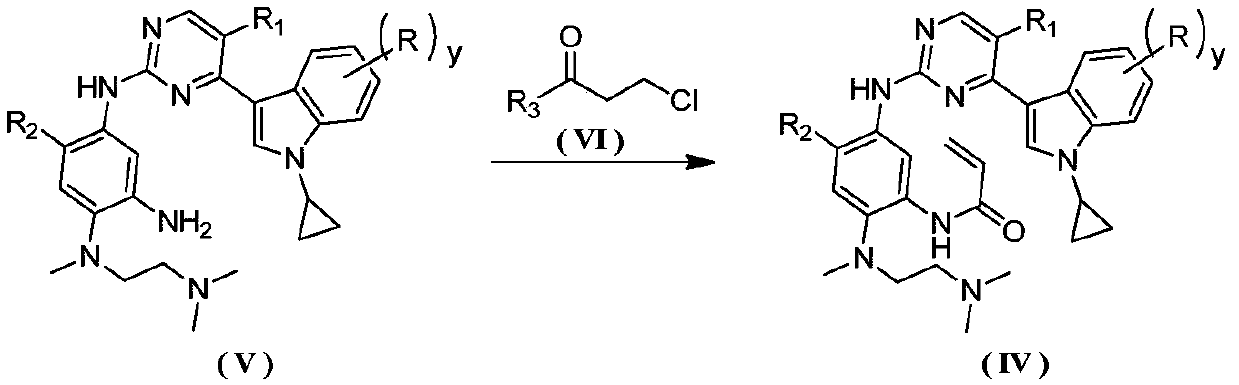
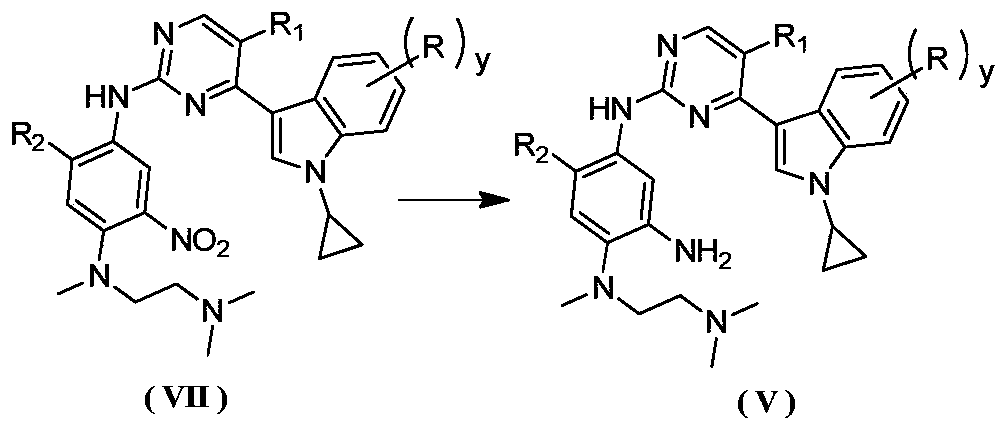
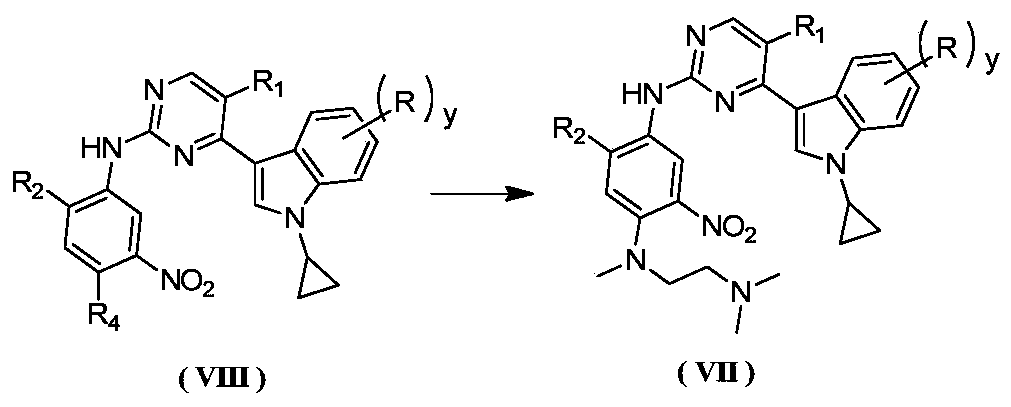
Preparation method for antagonizing drug-resistance anti-tumor EGFR (Epidermal Growth Factor Receptor) inhibitor
A technology of reagents and general formulas, applied in the field of drug synthesis, can solve problems such as unsuitable for large-scale production, increasing environmental pressure, and high irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] N-(5-((4-(1-cyclopropyl-1H-indol-3-yl)pyrimidin-2-yl)amino)-2-((2-(dimethylamino)ethyl)(methyl Preparation of base) amino)-4-methoxyphenyl)acrylamide mesylate
[0120]
[0121] The first step: the preparation of 3-(2-chloropyrimidin-4-yl)-1H-indole
[0122]
[0123] Add indole (236.0 g, 2.02 mol), tetrahydrofuran (1200 mL) into the reaction flask. Cool to 0°C, under nitrogen protection, slowly add methylmagnesium bromide (672 mL, 3 mol / L 2-methyltetrahydrofuran solution) dropwise into the system. After the dropwise addition was complete, stir for 1 hour. Add 2,4-dichloropyrimidine (120.0 g, 0.81 mol) and stir for 1 hour. Heat to an internal temperature of 70°C, stir the reaction at this temperature for 5h, stop heating, and cool to room temperature. Ethyl acetate (600 mL) was added to the reaction flask, followed by saturated aqueous ammonium chloride (1200 mL). Stir to separate the layers and save the organic phase. The aqueous phase was extracted with ethy...
Embodiment 2
[0149] N-(5-((4-(1-cyclopropyl-1H-indol-3-yl)pyrimidin-2-yl)amino)-4-(difluoromethoxy)-2-((2- Preparation of (dimethylamino)ethyl)(methyl)amino)phenyl)acrylamide
[0150]
[0151] The first step: 4-(1-cyclopropyl-1H-indol-3-yl)-N-(2-(difluoromethoxy)-4-fluoro-5-nitrophenyl)pyrimidine-2 - Preparation of amines
[0152]
[0153] 3-(2-Chloropyrimidin-4-yl)-1-cyclopropyl-1H-indole (80 mg, 0.29 mmol) and 2-(difluoromethoxy)-4-fluoro-5-nitroaniline (64mg, 0.29mmol) was dissolved in 2-pentanol, heated to microwave reaction for 1 hour, cooled to room temperature, evaporated to remove the solvent, and the residue was separated and purified by preparative thin layer chromatography to obtain 4-(1-cyclopropyl-1H -indol-3-yl)-N-(2-(difluoromethoxy)-4-fluoro-5-nitrophenyl)pyrimidin-2-amine (76 mg).
[0154] MS m / z(ESI):456.1[M+H] + .
[0155] The second step: N1-(4-(1-cyclopropyl-1H-indol-3-yl)pyrimidin-2-yl)-2-(difluoromethoxy)-N4-(2-(dimethyl Preparation of amino)ethyl)-N4-met...
Embodiment 3
[0170] N-(5-((4-(1-cyclopropyl-1H-indol-3-yl)pyrimidin-2-yl)amino)-2-((2-(dimethylamino)ethyl)(methyl Preparation of (yl)amino)-4-(trifluoromethoxy)phenyl)acryloylamide
[0171]
[0172] N-(5-((4-(1-cyclopropyl-1H-indol-3-yl)pyrimidin-2-yl)amino)-2-((2-(dimethylamino)ethyl)(methyl Base) amino) -4- (trifluoromethoxy) phenyl) acryloylamide The preparation method is similar to Example 1.
[0173] 1 H NMR (400MHz, CD 3 OD)δ9.56(s,1H),8.89(s,1H),8.56(m,1H),8.08(d,1H),7.71(d,1H),7.50(d,1H),7.32(m, 3H),6.96(m,1H),6.79-6.43(m,2H),6.09(dd,1H),5.85(d,1H),3.62(m,2H),2.75(m,3H),2.40-2.50 (m,3H),2.94(s,6H),1.24(m,2H),1.14(m,2H);
[0174] MS m / z(ESI):580.6[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com