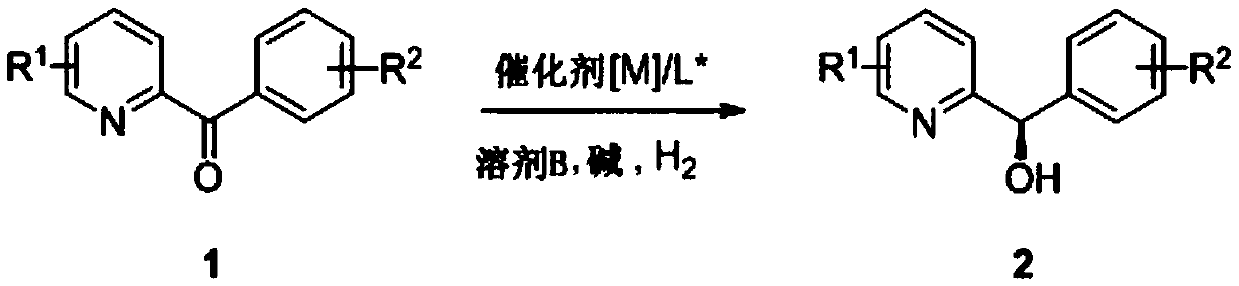
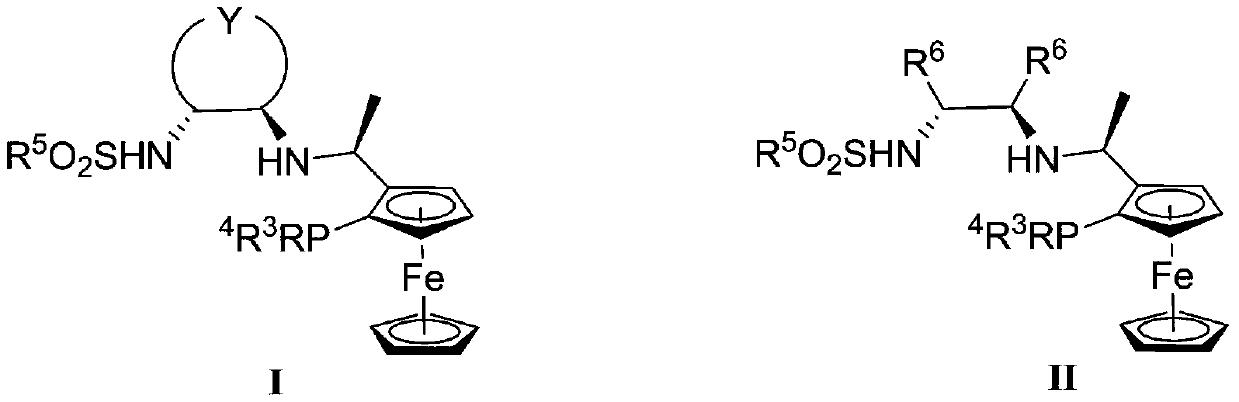
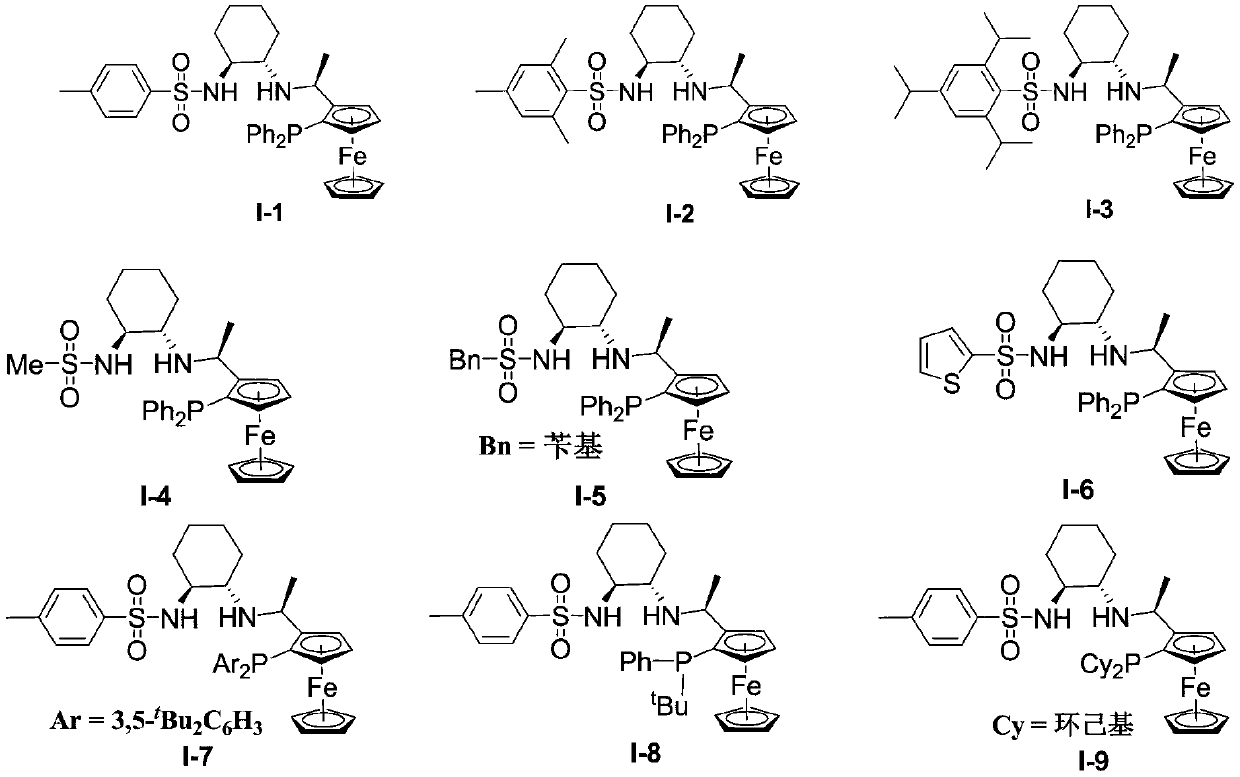
Preparation method of (R)-phenyl (pyridine-2-base) methanol derivative
A technology of derivatives and pyridine, which is applied in the field of preparation of phenylcarbinol derivatives, can solve the problems of unsatisfactory industrial production, single catalytic system, and poor substrate universality, and achieve great implementation value and social and economic benefits, three-dimensional Good selectivity and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation of (R)-phenyl(pyridin-2-yl)methanol
[0029] 1) The chiral ligand I-1 (16.6mg, 0.025mmol), metal complex [Ir(COD)Cl] 2 (8.0 mg, 0.012 mmol) was added to a reaction flask, methanol (1.5 mL) was added under argon atmosphere, and the reaction was stirred at 25° C. for 0.5 h to prepare a catalyst.
[0030] 2) Add phenyl(pyridin-2-yl)methanone (44.0g, 0.24mol) into the autoclave, add the catalyst prepared in step 1), potassium tert-butoxide (1.34g, 12mmol), methanol (100mL) , Charge H 2 (3.0MPa), react at 40℃ for 12h. After the reaction is over, the reaction solution is concentrated under reduced pressure to recover the organic solvent, then an appropriate amount of water is added, extracted with ethyl acetate, the organic phase is dried and desolventized to obtain (R)-phenyl (Pyridin-2-yl)methanol (42.6g, 0.23mol), yield: 96%, purity 97%, ee value 94%.
Embodiment 2
[0031] Example 2: Preparation of (R)-phenyl(pyridin-2-yl)methanol
[0032] 1) The chiral ligand I-1 (16.6mg, 0.025mmol), metal complex [Ir(COD)Cl] 2 (8.0 mg, 0.012 mmol) was added to a reaction flask, methanol (1.5 mL) was added under argon atmosphere, and the reaction was stirred at 25° C. for 0.5 h to prepare a catalyst.
[0033] 2) Add phenyl(pyridin-2-yl)methanone (44.0g, 0.24mol) into the autoclave, add the catalyst prepared in step 1), lithium tert-butoxide (0.96g, 12mmol), methanol (100mL) , Charge H 2 (3.0MPa), react at 40℃ for 12h. After the reaction is over, the reaction solution is concentrated under reduced pressure to recover the organic solvent, then an appropriate amount of water is added, extracted with ethyl acetate, the organic phase is dried and desolventized to obtain (R)-phenyl (Pyridin-2-yl)methanol (43.1g, 0.23mol), yield: 97%, purity 97%, ee value 99%.
Embodiment 3
[0034] Example 3: Preparation of (R)-phenyl(pyridin-2-yl)methanol
[0035] 1) The chiral ligand I-1 (16.6mg, 0.025mmol), metal complex [Ir(COD)Cl] 2 (8.0 mg, 0.012 mmol) was added to a reaction flask, methanol (1.5 mL) was added under argon atmosphere, and the reaction was stirred at 25° C. for 0.5 h to prepare a catalyst.
[0036] 2) Add phenyl(pyridin-2-yl)methanone (44.0g, 0.24mol) into the autoclave, add the catalyst prepared in step 1), sodium carbonate (1.3g, 12mmol), methanol (100mL), and charge Enter H 2 (3.0MPa), react at 40℃ for 12h. After the reaction is over, the reaction solution is concentrated under reduced pressure to recover the organic solvent, then an appropriate amount of water is added, extracted with ethyl acetate, the organic phase is dried and desolventized to obtain (R)-phenyl (Pyridin-2-yl)methanol (40.9g, 0.22mol), yield: 92%, purity 98%, ee value 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com