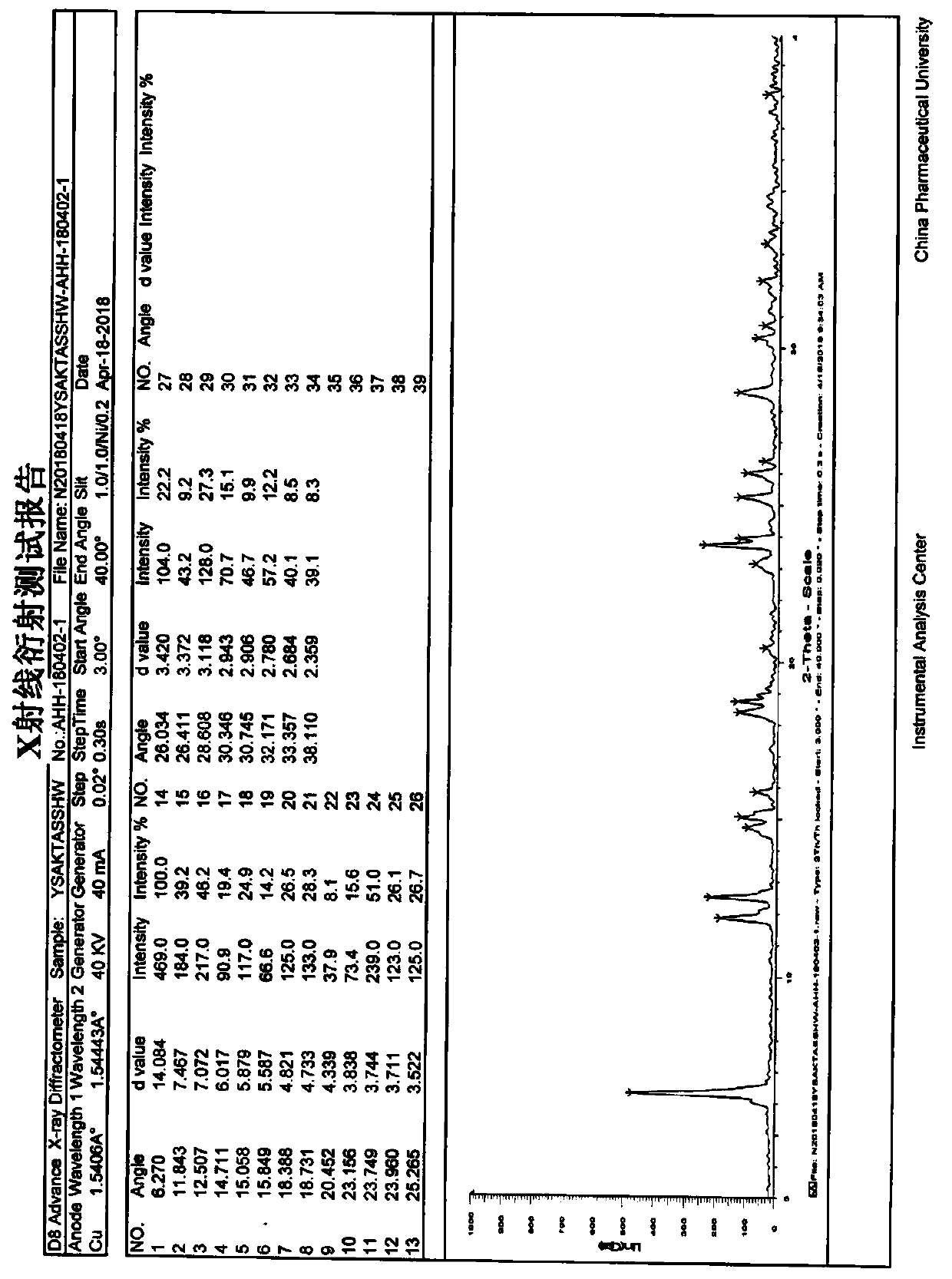
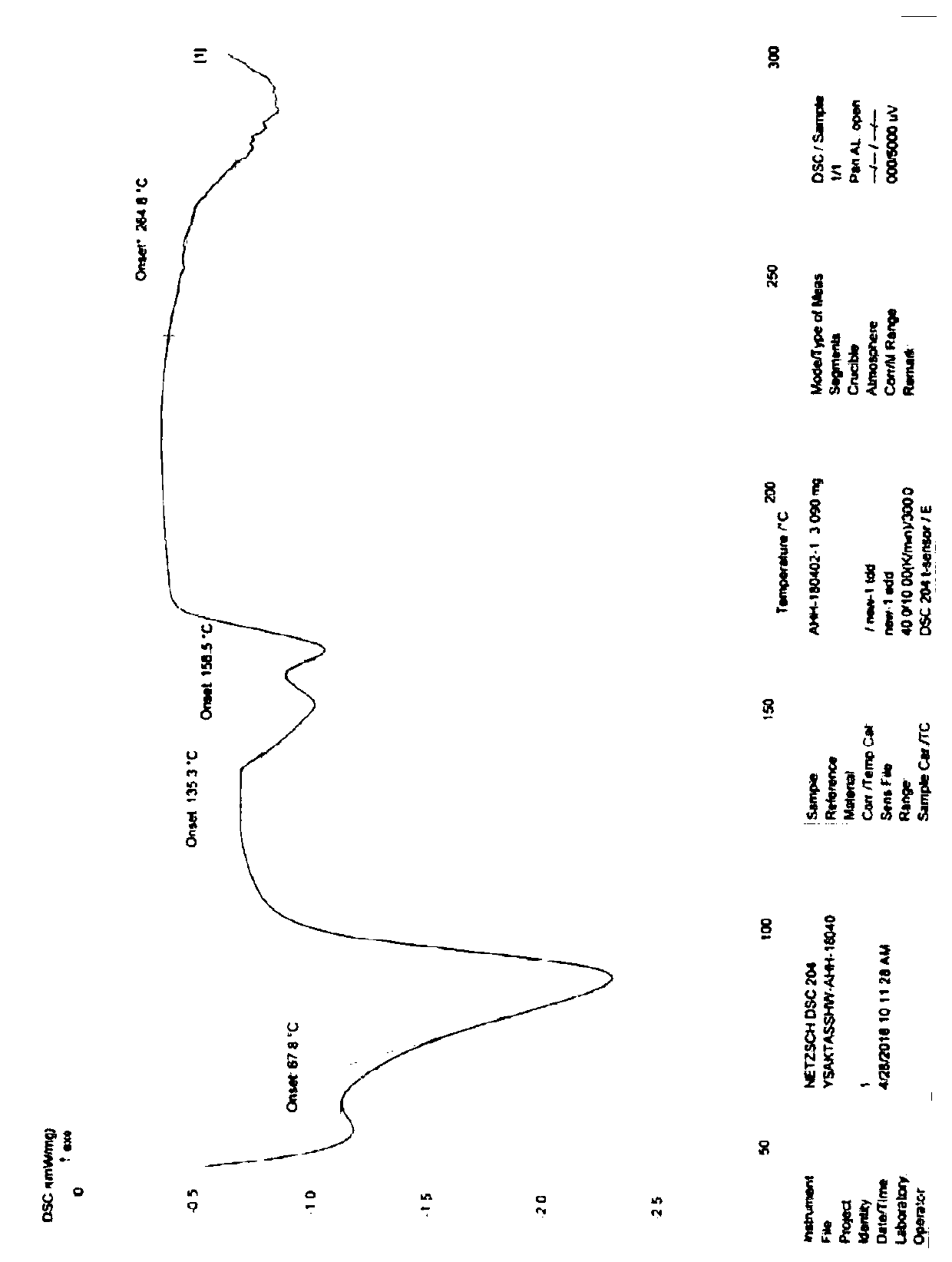
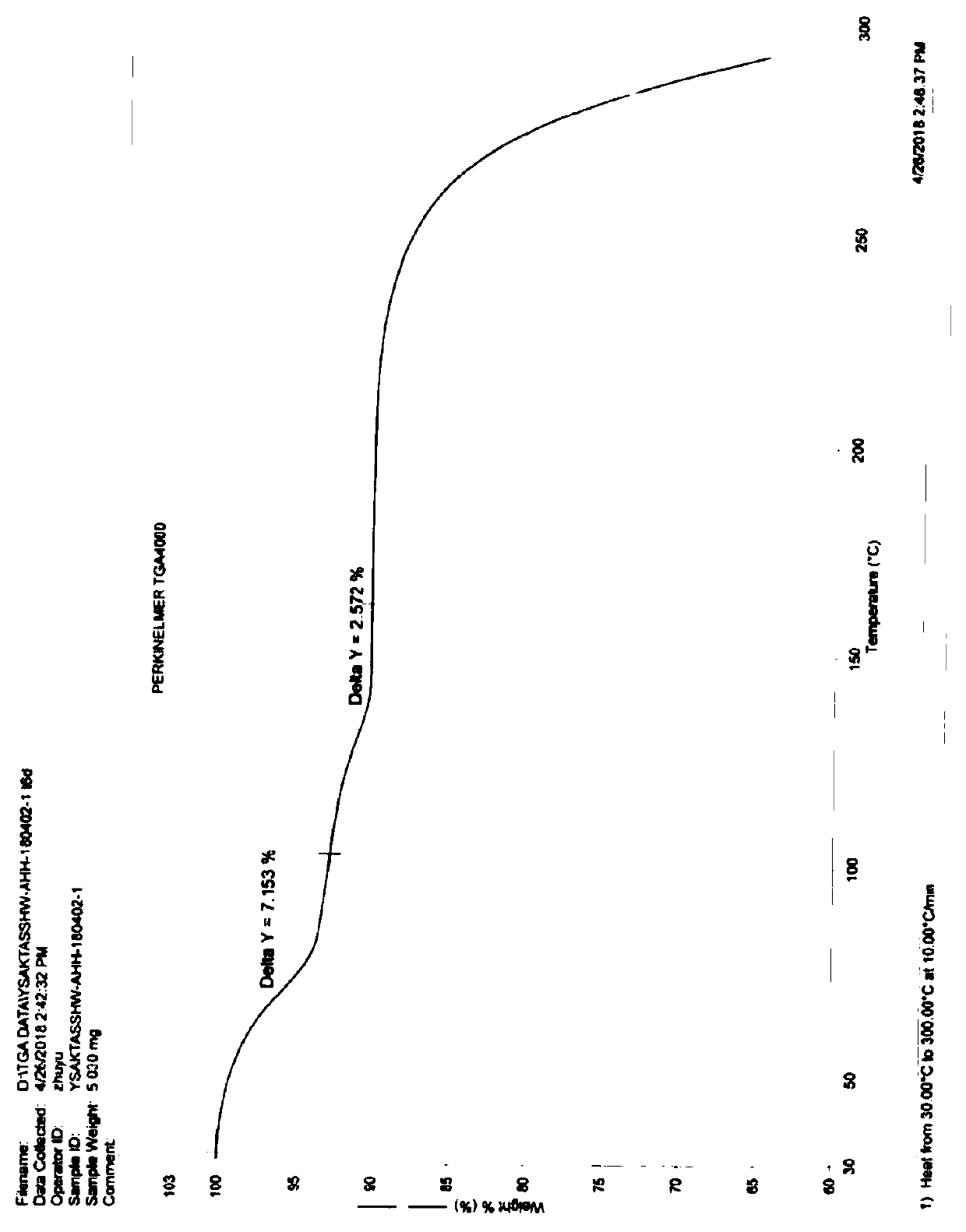
Industrial production method of acotiamide hydrochloride
The technology of acotiamide hydrochloride and production method, which is applied in the field of medicine and chemical industry, can solve the problems of low purity of acotiamide hydrochloride, unfavorable industrial production and high equipment requirements, and achieves simplified equipment requirements, low production cost and high response. Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
[0036] This embodiment is the preparation method of intermediate II, specifically as follows:
[0037] Add 100L of dichloromethane into a 300L reaction kettle, add 20.95kg of 2,4,5-trimethoxybenzoic acid under stirring, control the temperature at 20-25°C, add 16.22kg of oxalyl chloride dropwise, about 1.5 drops, The gas generated during the dropping process (mainly hydrogen chloride, carbon dioxide and a small amount of carbon monoxide) is absorbed by the tail gas absorption device, and the reaction solution changes from a white turbid solution to a yellow-green solution. Slowly add 0.85kg of DMF after dropping for about 0.5h. A large amount of gas (mainly hydrogen chloride, carbon dioxide and a small amount of carbon monoxide) generated during the dropping process is absorbed by the tail gas absorption device, and the reaction solution turns into a dark green solution. After dropping, keep it warm at 20-25°C for 1 hour.
[0038] The reaction solution was distilled under redu...
Embodiment 2)
[0042] This embodiment is the preparation method of intermediate III, specifically as follows:
[0043]Add 125L of DMF to a 300L reactor, then add 12.5kg of intermediate II prepared in Example 1, 11.83kg of pyridine hydrochloride and 5.4kg of pyridine under stirring, then heat up to reflux (about 145-150 ℃), insulated and stirred for 9 hours, and the reaction solution was a brown solution. After the reaction is over, stop heating and cool to 20-25°C for about 2 hours.
[0044] Add 210L of water into another 500L reaction kettle, stir and cool down to 0-5°C, then slowly add the above reaction solution into the reaction kettle, stir and crystallize at 15-25°C for 2h, and centrifuge.
[0045] Add 125L of glacial acetic acid and the filter cake obtained after centrifugation to another 300L reactor, stir and heat to reflux (about 115-120°C), dissolve and keep stirring for 2 hours. After the reaction, stop heating, cool to 20-25°C for about 2 hours, stir for another 30 minutes, ce...
Embodiment 3)
[0047] The present embodiment is the preparation method of acotiamide, specifically as follows:
[0048] First, prepare 10wt% sodium carbonate aqueous solution: add 125L of water in a 500L reactor, then add 12.5kg of Na 2 CO 3 Solid, stir to dissolve, store at 15-25°C until use.
[0049] Then, add 100L of toluene into the 300L reaction kettle, add 10.00kg of the intermediate III prepared in Example 2 under stirring, stir and heat to 70-80°C, and then dropwise add 34.98kg of N,N-diisopropylethylene di Amine, after dropping, heat to reflux (about 105-110°C), keep stirring and react for 4h. After the reaction is over, stop heating, cool down naturally to 20-25°C (about 2 hours), then slowly add 50L of glacial acetic acid dropwise, the temperature is controlled at no higher than 30°C, after the drop, the pH is 6.0-6.5, and quickly drops to 20 ~25°C.
[0050] Next, 75 L of water was added to the reactor for extraction and liquid separation. The water layer was transferred to an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com