Pyrazolopyridine aldehyde sulfite ion fluorescent probe
A technology of pyrazolopyridine aldehyde sulfites and fluorescent probes, which can be used in fluorescence/phosphorescence, luminescent materials, organic chemistry, etc., and can solve problems such as low application reporting.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
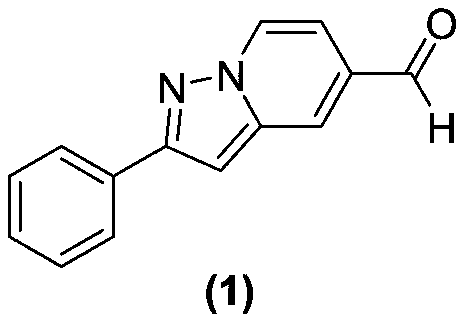
[0014] Embodiment 1: the synthetic scheme of formula (1) compound is shown in the following formula:
[0015]
[0016] Concrete synthetic steps are as follows:
[0017] Add 0.27g (1.00mmol) ethyl 2-phenylpyrazolo[1,5-a]pyridine-5-carboxylate, 0.38g (10.00mmol) sodium borohydride, 15mL ethanol successively in a 25mL round bottom flask, Reflux reaction at 80°C for 10 hours, pour into 100mL water, precipitate solid, filter with suction, and dry to obtain 0.20g colorless solid product 2-phenyl-5-hydroxymethylpyrazolo[1,5-a]pyridine, producing The rate is 89.3%.
[0018] H NMR spectrum, carbon spectrum determination: 1 H NMR (DMSO-d 6 ,400MHz),δ8.63(d,J=8.0Hz,1H),7.98(d,J=8.0Hz,2H),7.55(s,1H),7.47(m,2H),7.38(m 1H), 7.00(s,1H),6.81(dd,J=8.0Hz,4.0Hz,1H),5.42(t,J=8.0Hz,1H),4.54(d,J=8.0Hz,2H). 13 C NMR (DMSO-d 6 ,100MHz), δ157.74, 146.32, 144.23, 138.17, 133.96, 133.53, 131.15, 118.62, 116.70, 98.46, 67.12.
Embodiment 2
[0020]
[0021] Concrete synthetic steps are as follows:
[0022] 0.23g (1.00mmol) 2-phenyl-5-hydroxymethylpyrazolo[1,5-a]pyridine, 0.87g (10.00mmol) manganese dioxide, 15mL tetrahydrofuran, 70 The reaction was refluxed for 10 hours at high temperature, concentrated, and 0.13 g of colorless solid product 2-phenyl-5-formylpyrazolo[1,5-a]pyridine was obtained by column chromatography, with a yield of 58.6%.
[0023] H NMR spectrum, carbon spectrum determination: 1 H NMR (DMSO-d 6 ,400MHz),δ9.97(s,1H),8.52(d,J=8.0Hz,1H),8.03(s,1H),7.98(d,J=8.0Hz,2H),7.48(t,J= 8.0Hz, 2H), 7.41(m, 1H), 7.24(m, 1H), 7.10(s, 1H). 13 C NMR (DMSO-d 6 ,100MHz), δ189.66, 154.98, 140.36, 132.36, 131.76, 129.21, 128.94, 128.86, 126.51, 124.53, 109.99, 107.70, 98.03.
Embodiment 3
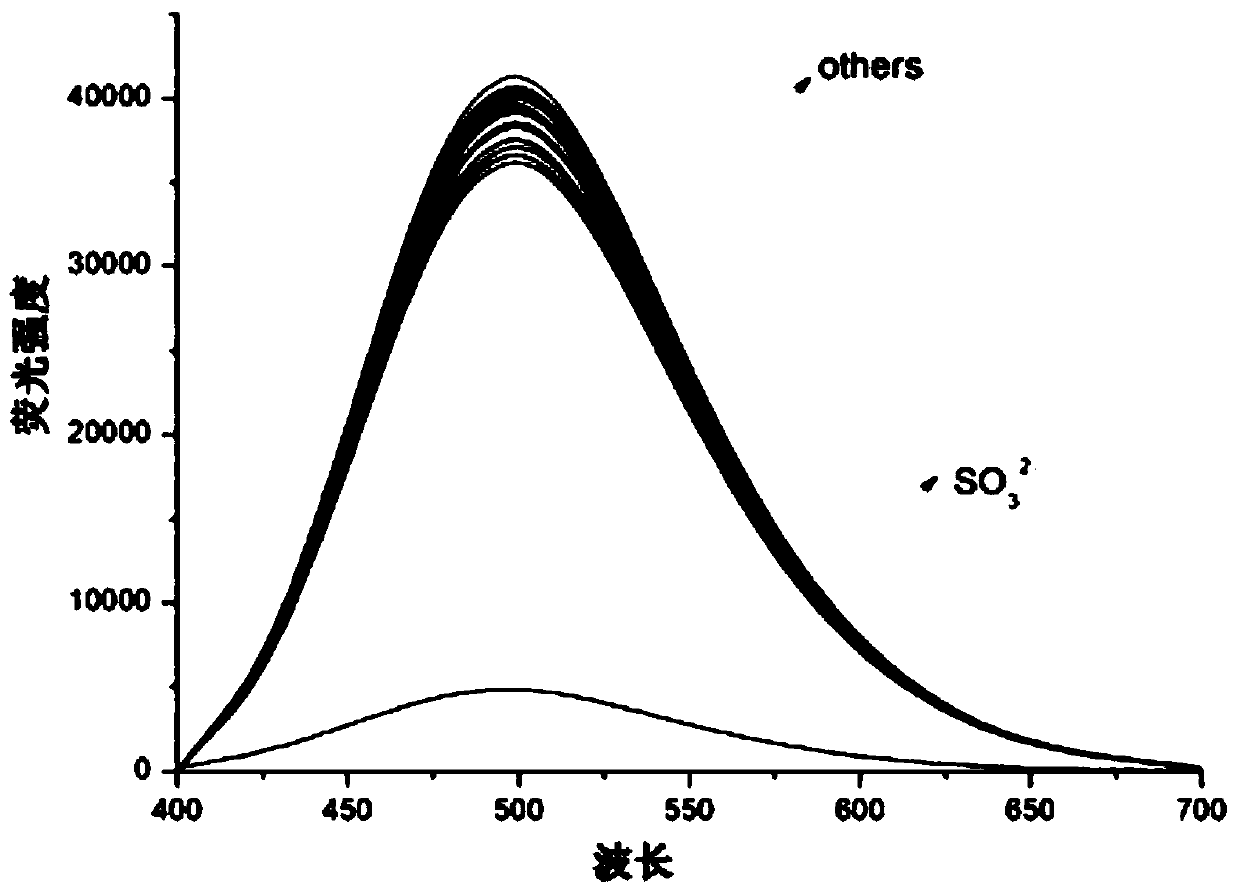
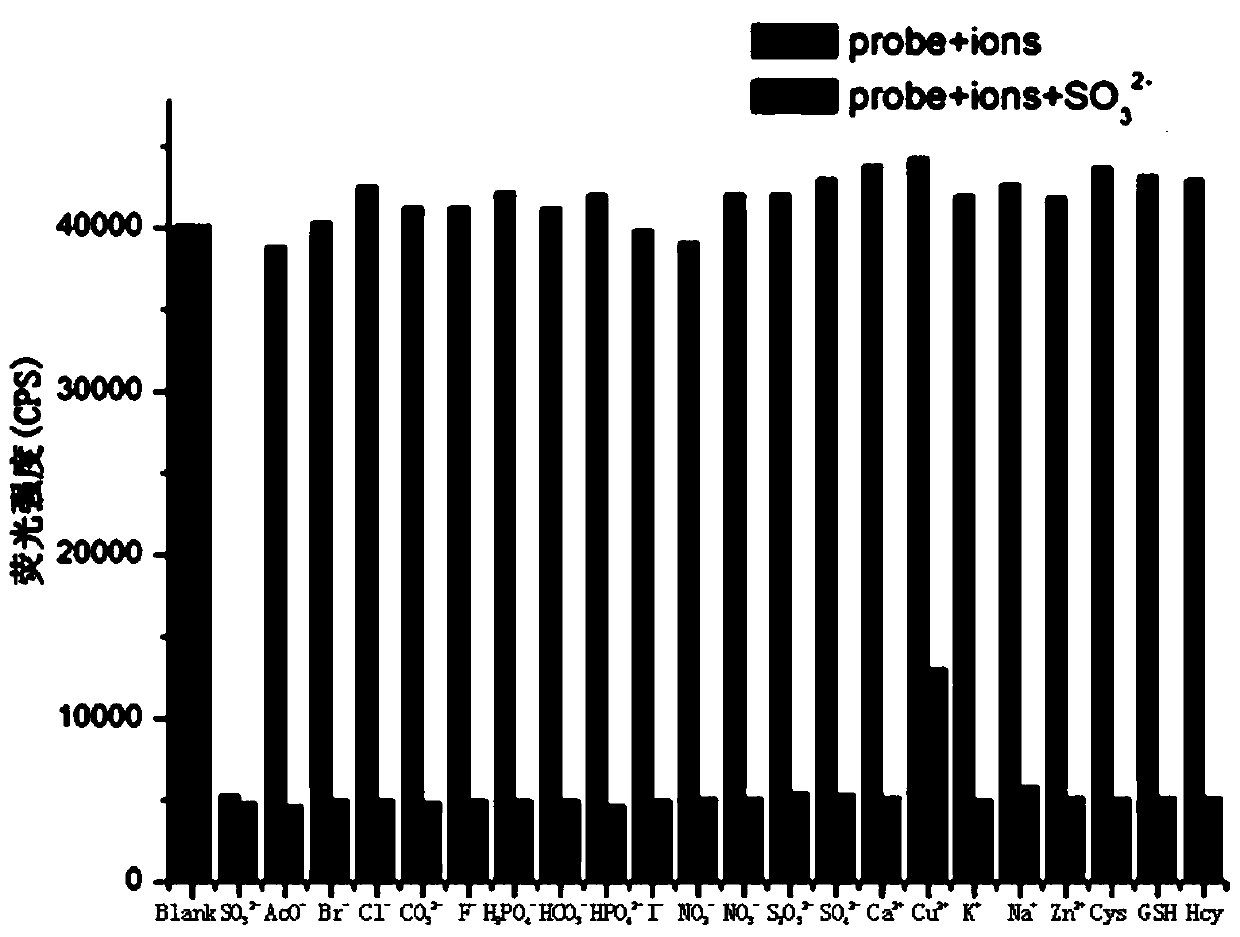
[0025] To formula (1) compound (10 -6 M) in the ethanol aqueous solution (ethanol: water=3:7) add the Na of 100 equivalents + ,Mg 2+ ,Zn 2+ ,Fe 3+ ,Cu 2+ ,F - ,Br - , I - , HCO 3 - , NO 3 - , ClO - , SO 4 2- , SCN - , S 2 o 3 2- , S 2- and SO 3 2- Afterwards, measuring its fluorescence emission intensity change at 500nm place finds: formula (1) compound is to SO 3 2- It has good fluorescence selectivity, and its fluorescence intensity is obviously weakened at 500nm, such as figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com